Precision for Medicine is part of the Precision Medicine Group, an integrated team of experts that extends Precision for Medicine’s therapeutic development capabilities beyond approval and into launch strategies, marketing communication, and payer insights. As one company, the Precision Medicine Group helps pharmaceutical and life-sciences clients conquer product development and commercialization challenges in a rapidly evolving environment.
How Translational Central Lab Services Facilitate Creative Solutions for Biomarker-Driven Studies

Biomarker-driven clinical trials are becoming the norm, with 16.5 percent of all trials and nearly half of oncology studies incorporating biomarkers (2019 to 2022).1 Across multiple therapeutic areas, the use of biomarkers is associated with a 15% higher likelihood of launch. However, biomarker translation can often be complex and always requires careful planning. There are myriad factors to be considered for successful execution of a biomarker-driven study, from deciding which biomarker(s) to use and how to measure them to determining the level of assay validation required and the feasibility of integrating that assay into a clinical trial.
In this article, we explore the importance of planning in biomarker clinical research and discuss common operational and scientific challenges associated with translational studies. We also share two case studies that highlight the need for creative solutions and coordinated central lab services when designing and implementing biomarker-driven trials.
Planning is paramount
The first step in designing a biomarker-driven study is deciding how that biomarker will be used, as the application of biomarker-derived data will dictate whether assay validation is required:
- Exploratory assays. These do not need validation but should be robust and reproducible. A key consideration is whether to do a batched assay at the end of the study or to perform the tests in real time or a half-way house of multiple small batches, perhaps by study cohort.
- Assays supporting clinical decision-making. If an assay is to be used to support patient enrollment or clinical decision-making, it may need to be validated under Clinical Laboratory Improvement Amendments (CLIA) or Good Clinical Laboratory Practices (GCLP). This higher level of validation and the potential need for reagent generation will take time.
- Assays supporting primary and secondary endpoints. Since this data will be reported to regulatory agencies, these assays must be validated, and an appropriate validation level must be determined.
Receptor occupancy (RO) assays are a common example demonstrating the importance of planning. With RO assays, which aim to assess how much of the target receptor is bound by drug, it is critical to define how the sample is collected, how and when it will be assayed, and how to calculate total receptor expression. Since the assay is typically performed on whole blood and needs to be performed as close as possible to draw, sample logistics are critical. The ideal scenario is to have all samples tested in a single lab, as RO assays are often flow cytometry-based and difficult to standardize. Given these constraints and variables, detailed planning is critical to setting up the assay for success.
Translational clinical studies are complex
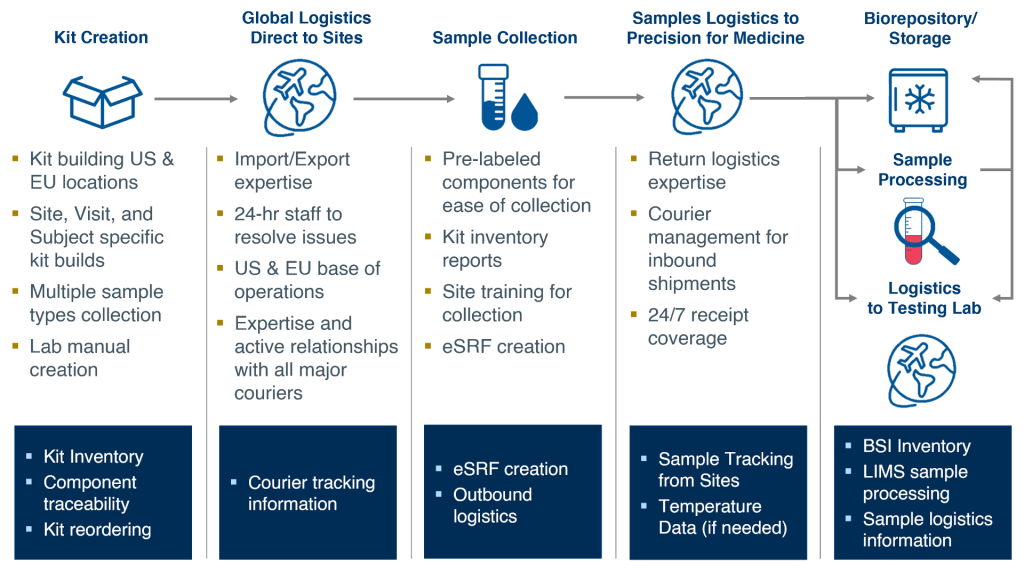
Clinical development is a significant investment, not just in dollars but also in effort and expertise. Studies generally involve multiple sites, each of which requires the same level of management regardless of enrollment. These sites collect samples and data that feed into a central lab, which then dispenses samples to multiple screening or specialty labs. Each of these nodes requires contracting, sample collection kits, and chain of custody traceability, and all generate and report data, which may be housed in disparate systems. The result is a complex and disconnected ecosystem (see Figure 1).
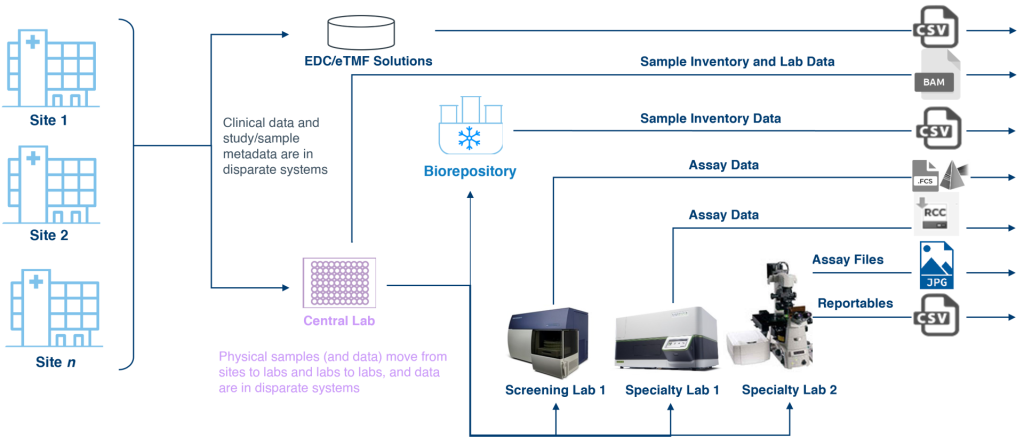
Execution of a translational clinical study is guided by the clinical trial protocol, which documents what is expected to happen, and the informed consent form (ICF), which memorializes what has been consented to by participants. To deliver on designed outcomes, sites need to have sample collection supplies on hand and the necessary training to collect samples in accordance with the protocol and ICF. The samples are handed off to couriers that can transport the samples from the clinical site to the central lab in a manner that is visible, traceable, and suitable for the sample requirements with respect to time, temperature, condition, and applicable regulations. The central lab accessions, stores, and distributes the samples for testing and, eventually, storage.
At every control point—the site, the courier, the central lab, the screening and specialty labs, and the biorepository—chain of custody must be maintained and appropriately documented, and sample data is generated. Managing and tracking study samples over their lifecycle can lead to both operational and scientific challenges:
- Courier management. Depending on where a trial is being executed, multiple couriers with specialty regional access may be required.
- Supply/kit inventory management. Processes need to be established for knowing that sites have sufficient supplies and kits on hand to perform scheduled collections as assigned within the protocol.
- Sample processing quality. If multiple labs are used, it is critical to ensure consistency in processing across all labs to generate quality data.
- Data harmonization. With so many nodes and control points, systems are often disconnected, and multiple technologies may be needed for managing, integrating, and harmonizing source data.
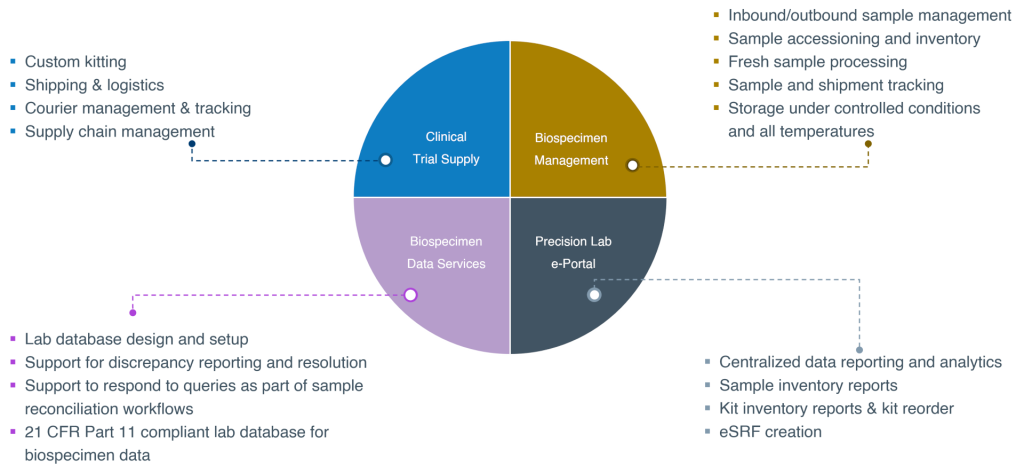
For translational clinical studies, central lab services need to operate at the intersection of clinical trial operations, translational science, and technology to provide insight into the full sample lifecycle, from upstream sample collection to downstream sample testing (see Figure 2).
Facilitating biomarker research: Case studies
Case Study 1: Developing a receptor occupancy assay for a first-in-class monoclonal antibody
A sponsor with a first-in-class monoclonal antibody (mAb) targeting a co-stimulatory molecule highly expressed on CD4 and CD8 T cells, as well as some expression on other cell types, wanted to develop an RO assay for monitoring target engagement and changes in receptor levels. The first challenge of assay development was the need to assess multiple cell types, as early data suggested that not only was the target receptor expressed differently on different cell types, engagement of that receptor could also have different pharmacokinetics (PK) and a different response. Consequently, a more extensive flow panel with 10 colors was needed for gating different cell types.
The second challenge was associated with the dispersed geography of the study, with clinical sites in the US, New Zealand and Australia. Given the complicated flow assay and analysis, it was determined that running the study out of single lab would be optimal for data quality. However, time course studies on titrating the mAb into whole blood showed that samples would need to be tested within 48 hours of collection, making transit to a single lab not feasible. Peripheral blood mononuclear cell (PBMC) isolation was tested as a potential solution, as these cells could be cryo-preserved, but this approach adversely impacted RO data.
The second approach tested involved the use of fixatives for stabilizing whole blood. There are many fixatives available, which vary in ease of use and marker compatibility. Consequently, it is critical to perform feasibility studies to evaluate how the fixative modulates the resulting data. For this RO assay, different stabilization protocols were tested:
- Cyto-Chex™ BCT tubes were not compatible with all markers in the panel
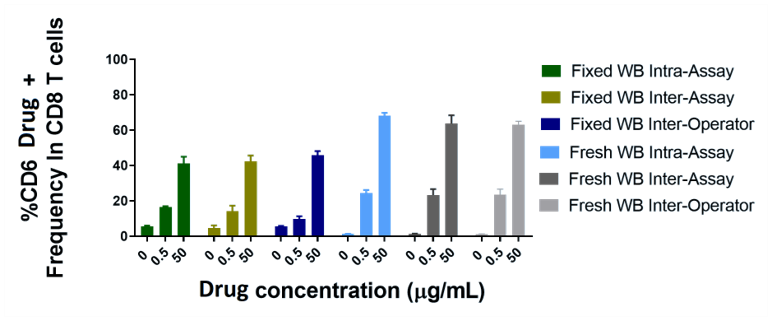
- Stabilization results with Smart Tube™ Proteomic fixative correlated with intra-assay, inter-assay, and inter-operator data from fresh whole blood at three different drug concentrations, though the total dynamic range was somewhat constrained (see Figure 3). Stability testing showed at least 120 days of stability.
The selection of Smart Tube™ Proteomic fixative created a third challenge since this stabilizer comes in a bottle, rather than a vacutainer, which would be difficult to implement at clinical sites. The solution was building a custom kit with pre-aliquots of fixative and actively engaging with and training every site to ensure they understood how to use the fixative and store the stabilized sample. Since the samples can be stored long-term at −80 °C, they could be shipped to a single lab and run in a batch to control data consistency.
Case Study 2: Maximizing the scientific value of a single sample
To minimize patient burden and maximize scientific value, creative approaches may be needed to derive the maximum amount of actionable data from a single sample. With proactive planning, it is possible to optimize downstream assays from minimal amounts of tissue. In this case study, a skin punch biopsy was used for three different assays by first placing it into RNAlater® and then dividing the sample into thirds. The assays performed were:
- H&E staining and multiplex immunofluorescence on a formalin-fixed paraffin-embedded (FFPE) block, the results of which showed that overall structure and quality of the biopsy was preserved and markers correlated with tissue that was immediately fixed and embedded without RNALater treatment.
- RNA extraction with RNA-Seq, which demonstrated sufficient RNA yield to allow successful library construction.
- PCR-based epigenetic immunophenotyping with Epiontis ID, which looks at methylation status of promoters on specific genes.
Epiontis ID is an ideal tool for this application because, unlike real-time flow cytometry, it is not time-sensitive, does not require viable cells, and utilizes only minimal sample volume. Moreover, it is an operator-independent technology that produces precise and reproducible results.
An end-to-end central lab services solution
Involving a translational central lab service provider in translational study planning, design, and execution can help address the challenges associated with these trials. Experienced translational central labs can assist with biomarker selection, assay development and validation, and ideation of creative solutions for optimizing the feasibility—and maximizing the utility of—samples collected for biomarker interrogation. Ideally, they will serve as a single-source, end-to-end solution, managing everything from custom kit creation and deployment to sample collection, logistics, and storage.
At Precision for Medicine, we partner with sponsors to provide custom solutions that provide real-time visibility into the entire sample lifecycle, ensuring the biomarker-driven studies stay on track and generate robust, quality data.
Learn how we can help you maximize insights into patient biology in translational clinical studies >
References
1. Citeline data for trials with an actual or anticipated start date from January 2019 to December 2022.
-
Deborah Phippard, PhD
Pharma industry veteran and expert at biomarker-driven clinical trial design and execution. Leader of biomarker and drug development programs for pharmaceutical and diagnostics companies, as well as the National Institutes of Health. Spearheaded the discovery of pharmacodynamic biomarkers and novel targets for inflammatory disease therapy.
-
Joseph Neal
Joseph Neal is a biopharmaceutical industry leader noted for applying cross-functional resources to highly technical issues. Impressive track-record in supply chain strategy, manufacturing operations and support, new facility design and start-up, tech transfer, and R&D process development. Equally capable of driving shop floor tasks and strategic objectives through senior levels.