Reaching your R&D goals is challenging in the COVID-19 environment. These insights from the frontlines can help. View the webinars on-demand now.
Reaching your R&D goals is challenging in the COVID-19 environment. These insights from the frontlines can help. View the webinars on-demand now.
ON-DEMAND WEBINAR:
Applying Mechanistic Modeling Approaches to COVID-19
As the COVID-19 pandemic progresses, the urgency to find effective treatments intensifies. The concept of drug repurposing—that drugs approved for one condition may treat another—is not new. The challenge is predicting which drugs are most likely to be effective. Advances in computational biology and translational informatics can accelerate the process of evaluating a compound’s MOA in various indications and patient populations. In the context of a global pandemic, a therapy approved even weeks more quickly can have a positive impact on human life and the global economy.
This webinar provides understanding of:
- Predictive analysis of drug response for repurposing or indication expansion
- Investigation of mechanism of action for potential therapeutic agents
- Analysis of preclinical model recapitulation
- Co-therapeutic target pathway selection
Learn more about Precision’s translational and computational biology solutions
ON-DEMAND WEBINAR:
Overcoming Remote Monitoring Challenges in the Face of COVID-19
The social distancing required by COVID-19 has created unique challenges for R&D programs, including the monitoring of clinical trials. In response, innovative solutions have arisen to continue to connect with patients, get them access to treatments, and record their progress—all while meeting FDA and EMA requirements to document any discrepancies. Overcoming these challenges takes a unified approach.
Learn how this approach:
- Enables early detection of discrepancies with dashboard alerts and a collaborative interface
- Captures information regulatory agencies require for documentation of COVID-19 impacts
- Provides forward-looking visibility into expected sample collection, including evolving sample schedules
- Delivers quick insights through rapid deployment
Learn more about Precision’s clinical data management services
ON-DEMAND WEBINAR:
Strategically Accelerate COVID-19 Diagnostics From Development to Market
Many COVID-19 diagnostics programs have been delayed or derailed because of unanticipated challenges. COVID-19 success will require overcoming a variety of obstacles—from assay design and regulatory hurdles to sample sourcing and reimbursement considerations. This webinar explores real-world examples that will help you anticipate pitfalls, mitigate risks, and accelerate development.
Understand how to:
- Build a biospecimen foundation for diagnostic development and 510(k) submission
- Navigate the regulatory landscape for serology tests, from EUA to 510(k)
- Leverage important design considerations for market access
Precision for Medicine’s R&D Services
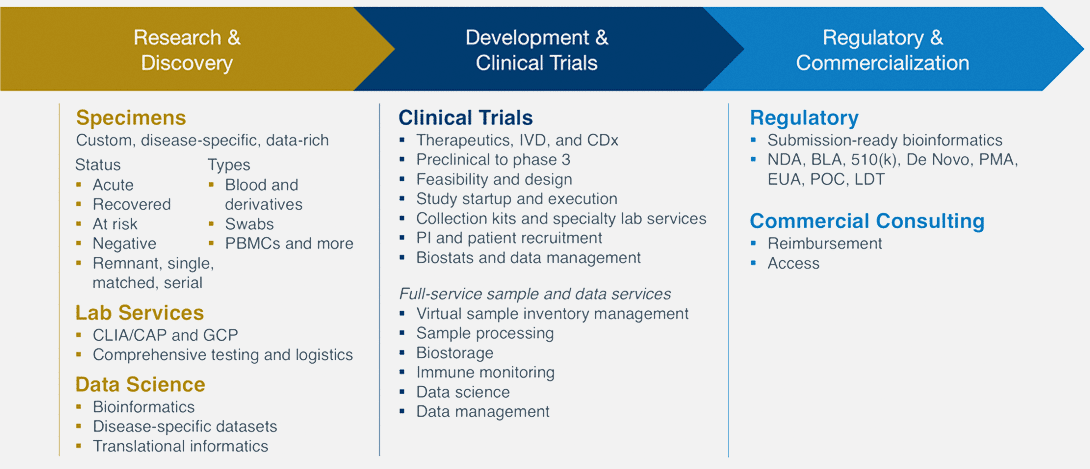
To learn about Precision for Medicine COVID-19 services click here.
Precision for Medicine’s R&D Services

To learn about Precision for Medicine COVID-19 services click here.
Tell us about your project requirements
Access to powerful, proprietary bioinformatics and computational biology tools is just one way Precision can advance your clinical development program. We can do so much more.
Tell us about your project requirements
Access to powerful, proprietary bioinformatics and computational biology tools is just one way Precision can advance your clinical development program. We can do so much more.




