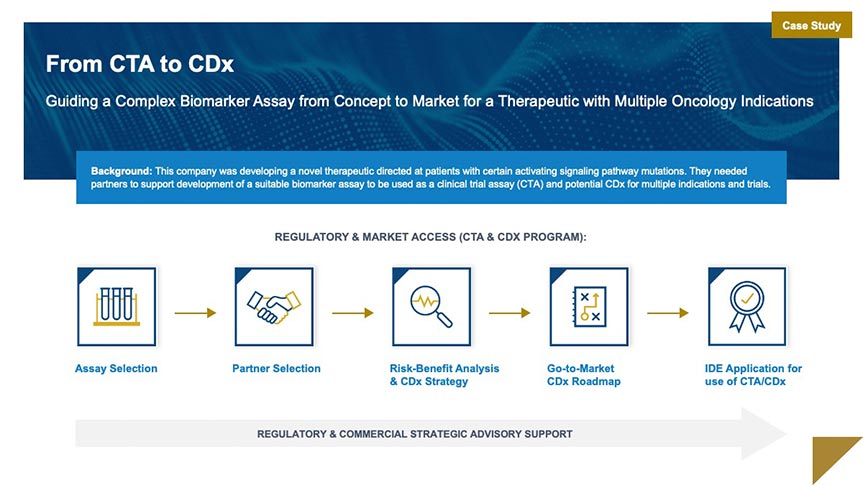
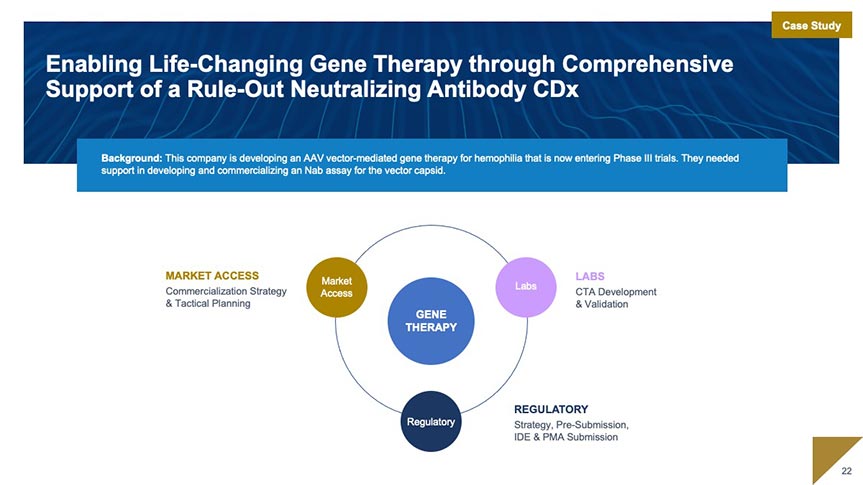
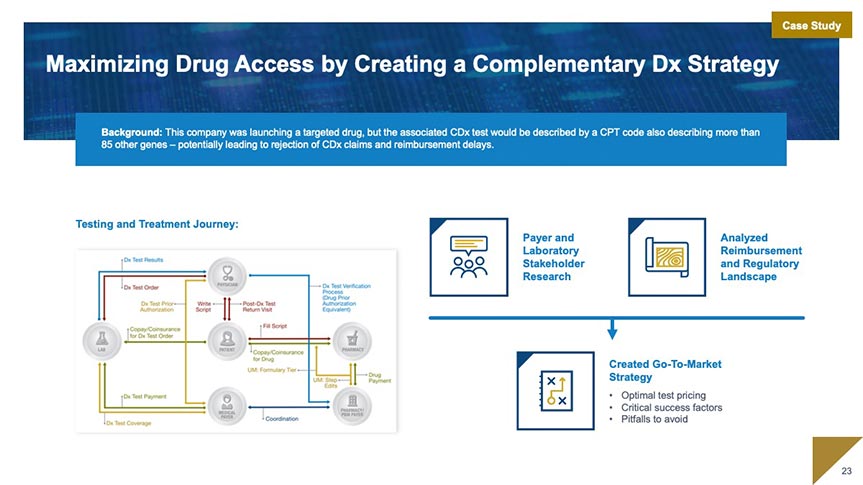
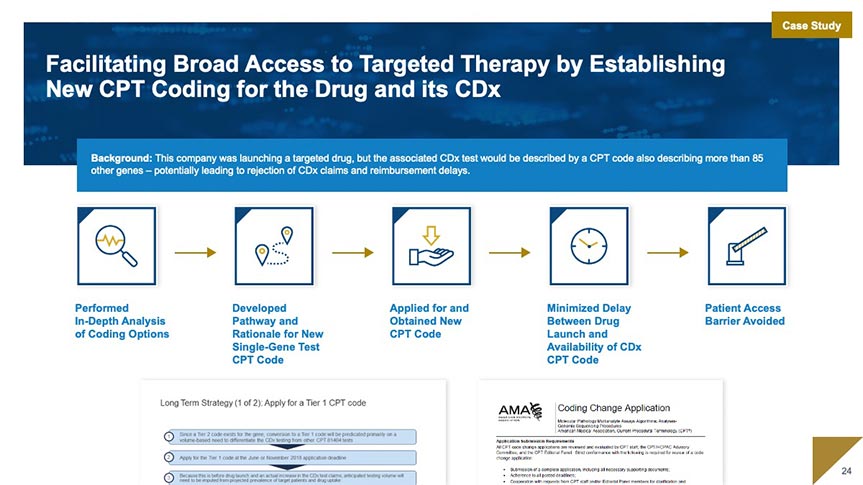
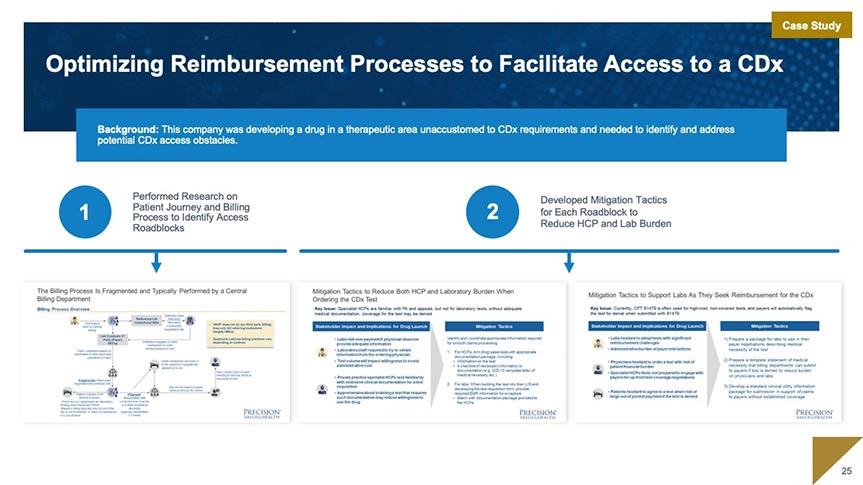
Market Access
Solutions for LDTs,
IVDs, and CDx
Our market access team works with you to develop and execute market access strategy beginning as early as assay development. We specialize in market access planning and strategy; coding, coverage, and payment; and evidence generation for coverage.
Accelerate Diagnostic and
Companion Diagnostic Commercialization
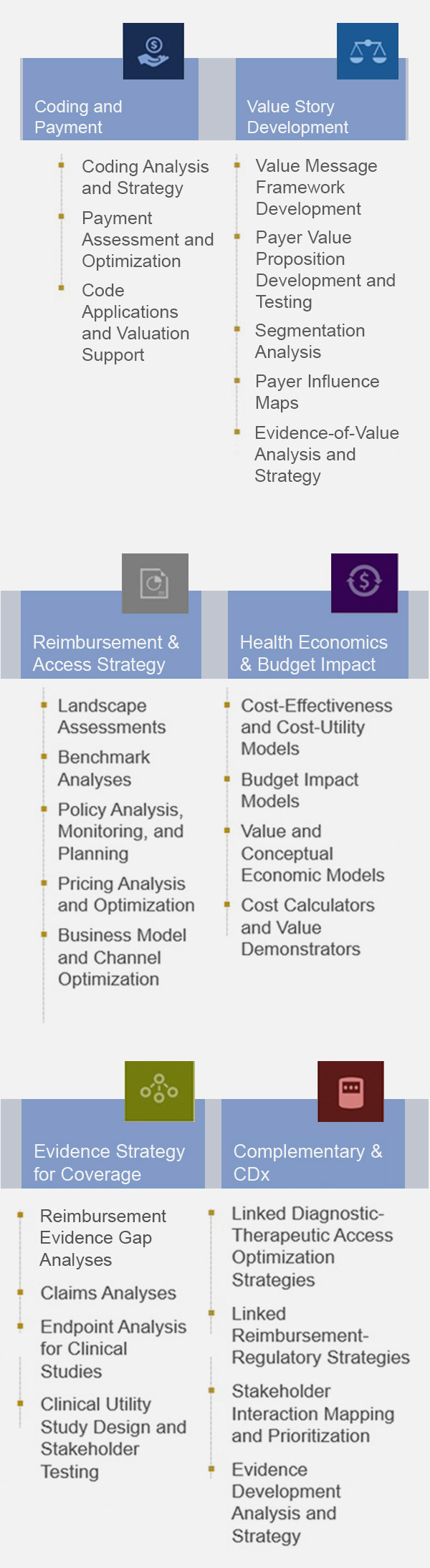
Market Access and Commercialization Strategy and Support
- Access and Commercialization Strategy: Landscape Analysis and Access Planning
- Coding, Coverage, and Payment: Code Creation and Valuation; Value Dossiers; Payer Presentations and Managed Markets Tools
- Evidence Strategy for Coverage: Clinical Utility
Market Access and Commercialization Strategy and Support Experience

Going Beyond Viral Detection:
COVID-19 Diagnostics
Conference: Tri-conference
Speaker: David Parker, PhD
View Our Market Access Case Studies
for Diagnostics and CDx
Biospecimens to Support Your Diagnostic Development
![]()
Blood, Biofluids, and Derivatives
Diseased and healthy human blood, plasma, serum, CSF, stool, ascites fluid, saliva, urine, and more.
![]()
Tissues
Pathologist-verified, fresh, frozen, and fixed tissue specimens from healthy and diseased human subjects.
![]()
Liquid Biopsy
Comprehensive services including kitting, collection, processing, and profiling from your patients or ours.
![]()
Custom Biospecimen Collections
Global clinical network, regulatory approved, and ready to enroll.
![]()
Viable Cells
HLA-typed cellular products including PBMCs, BMMCs, Leukopaks, DTCs, and more.
Get Critical Market Access Support From Concept to Commercial. Maximize Your Product’s Potential and Achieve Your Commercial Goals
Services That Complement Market Access Services: Biospecimens, Regulatory, and Custom Assays
Biospecimens
Thousands of IRB-approved, clinically-annotated biospecimens ready to ship, same day, to your lab. Whether using specimens for assay development, bench research, or generating big data for your research or development studies, we deliver.
Regulatory
Comprehensive regulatory planning and strategy, early agency interactions, design of analytical studies, and regulatory submissions. We can help you work with the FDA, EMA, MHRA, NMPA, and PMDA, and design a regulatory solution.
Custom Assays
Development of de novo assays with a particular specialization in cell-based assays and epigenetic immune cell phenotyping using our proprietary and patented Epionitis ID platform.