Immune Monitoring by Flow Cytometry
Immune Monitoring by Flow Cytometry
Flow cytometry services for immune response insights
Comprehensive approaches for your clinical or preclinical study
Applications
We provide a full range of flow cytometry assays including, but not limited to:
- Immunophenotyping*
- Receptor occupancy (RO) measurement
- Phospho-flow cytometry
- Tetramer assays
- CAR-T cell characterization for cell therapy development
- Cell sorting (FACS)
- ISO/GCP/GLP/CAP/CLIA regulated assays
- CLIA assays (BD FACSCanto™ Instruments)
*Many immunophenotyping studies can be cost-effectively accomplished with our proprietary Epiontis ID technology. Visit the Molecular Phenotyping page or Contact Us to learn more.
Instruments
All of our flow cytometry studies are conducted on BD Biosciences systems; we have multiple redundant instruments in laboratories across the globe.

BD LSRFortessa™
- 20 parameters
- 18 colors

BD FACSCanto™
- 12 parameters
- 10 colors
- Supports CLIA assays

BD FACSAria™ Fusion
- 20 parameters
- 18 colors
- 4-way sorting
- Tubes, 96-well plates, 384-well plates

To flow or not to flow: Immune cell profiling in translational research
Learn about key technologies available for immune cell profiling and the benefits and limitations of each approach.
Integrating biomarker and clinical trial data
To help researchers understand and interpret biomarker assay data in the context of other assays as well as the clinical trial data, Precision can deliver flow cytometry and other biomarker data using our proprietary QuartzBio platform.
QuartzBio features specific flow cytometry capabilities including interactive gating and visualization of high-dimensional data.

Global reach, multi-site support
We provide flow cytometry services that support pre-clinical and clinical research, including multi-site studies, conducted anywhere in the world. Samples are processed within 24 to 30 hours of draw and utilize standardized SOPs to ensure sample integrity.
Working with Precision
Precision scientists take a collaborative and consultative approach to projects and can provide recommendations on assay strategies and implementation, if needed. Services can be provided individually or as part of a comprehensive therapeutic development package including biomarker assays and clinical trials.
Scientific Poster: Development and technical validation of tetramer staining for use as a biomarker for assessing gluten-specific T cells in clinical studies
Presented at the 18th International Celiac Disease Symposium • 4–7 September 2019 • Paris, France
Gluten challenge in subjects with celiac disease results in a transient upregulation of gliadin-specific CD4+ T cells in the blood. Despite the increase, these cells are quite rare requiring a selective and sensitive assay for detection.
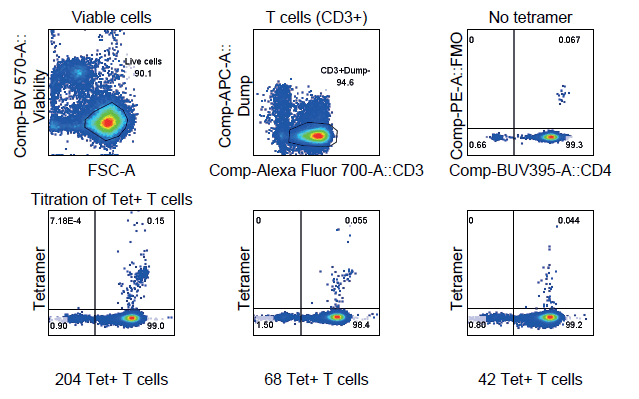
We have developed a 12-color tetramer flow assay to enable detection and immunophenotyping of the gliadin α-I/α-II CD4+ T cells in the blood.
Conclusions
This gliadin α-I/α-II DQ2 tetramer flow cytometric assay was developed and validated to be sensitive and selective. Technical validation of the assay was successfully met and the assay performs within the precision parameters.
This assay will enable characterization of the gliadin α-I/α-II CD4+ T cells in the blood for celiac patients, providing a more comprehensive evaluation of response to new therapies and may reduce invasive biopsy-based measurements.
Discuss your project requirements
Whether you know exactly the type of immune monitoring approach you need or would like expert support with project design, we can deliver.
Discuss your project requirements
Whether you know exactly the type of immune monitoring approach you need or would like expert support with project design, we can deliver.