COVID-19 diagnostics marketed under Emergency Use Authorization (EUA) are transitioning to comply with US FDA 510(k)/de novo requirements to remain on the market in the United States. Precision for Medicine experts are here to guide you through the process for clearance.
Regulatory, Clinical, and Quality Steps Required to Move From EUA to 510(k) Clearance
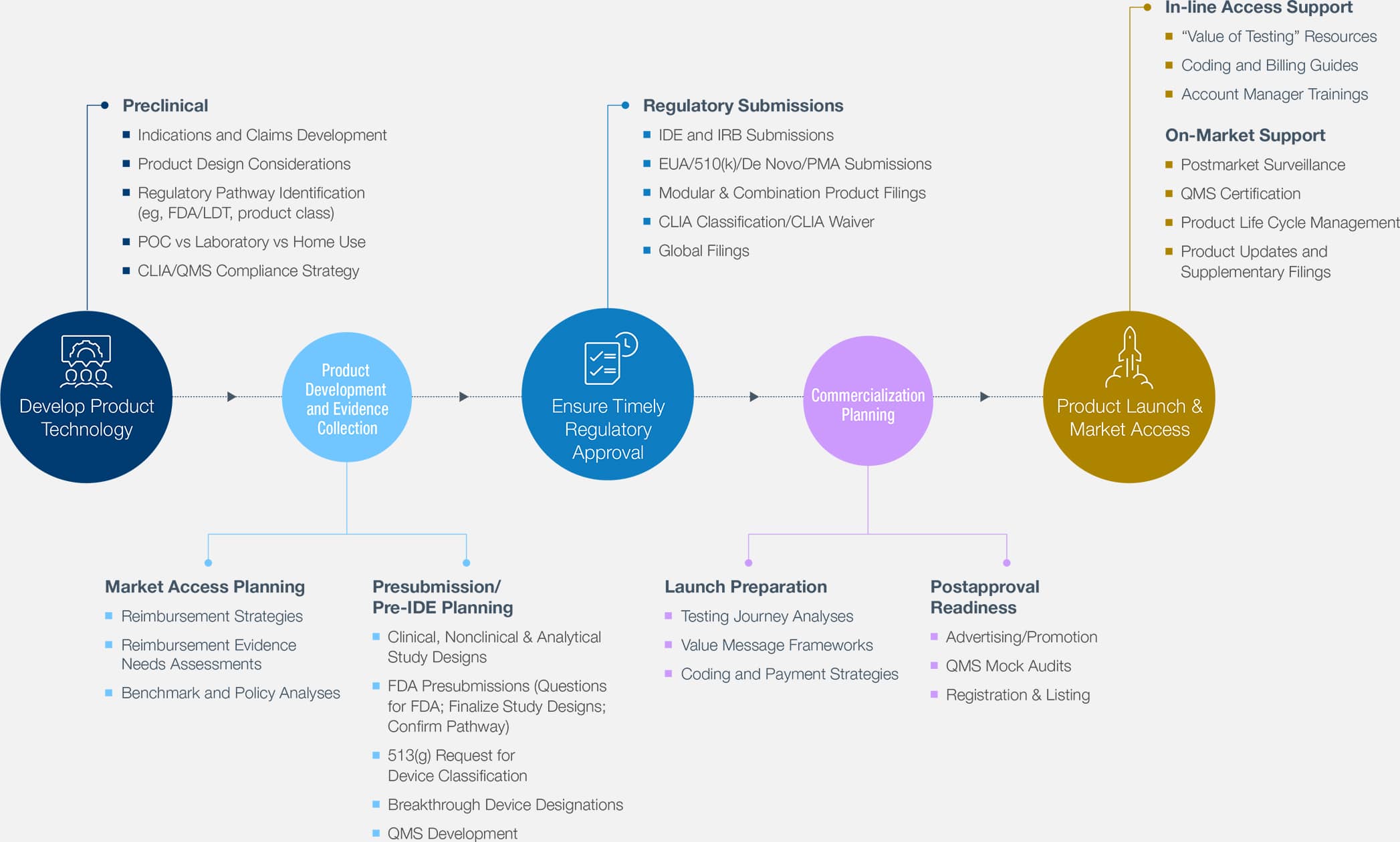
Since the emergence of the COVID-19 pandemic, an intense research effort has been focused on swiftly bringing diagnostic tests to market under EUA. Now, with the availability of 3 COVID-19 vaccines, we are seeing a steady increase in population immunity. Although the COVID-19 Public Health Emergency remains in effect, the FDA has already issued draft guidances on transition plans for devices under an EUA. Therefore, we expect manufacturers of diagnostics with EUAs to start transitioning to marketing submission, as the FDA has granted the first de novo and cleared the first molecular 510(k). A de novo has not been granted for an antigen or over-the-counter test.
With hundreds of COVID-19 assays with EUA potentially transitioning to 510(k) clearance in a short period, plans should now be made for the necessary analytical and clinical validation studies and regulatory submissions.
Precision for Medicine supports the efforts of companies developing COVID-19 diagnostics with:
- COVID-19 biospecimens sourced from our clinics and biobank
- Deep practical experience with COVID-19 EUAs, and 510(k) clearances for diagnostic tests
- Broad expertise in evolving US and EU diagnostic regulations
- Comprehensive lab support for immune monitoring, antibody characterization, and related research
Learn more about how Precision for Medicine can help you bring COVID-19 diagnostics to market – and ensure that they stay there. Contact us today.
Precision’s Unmatched Experience in Diagnostic Development Takes Your COVID-19 Product From Bench to Bedside
To learn more about our COVID-19 biospecimens offerings, visit http://www.precisionbiospecimens.com/covid-19/ or contact us.
Speak With an Expert About Your EUA Transition
With hundreds of Emergency Use– authorized COVID-19 assays potentially transitioning to 510(k) clearance in a short period, you should plan now for the necessary validation studies and regulatory filings.
Regulatory Strategy & Submissions Experience
100+
IVD pre-submissions, preclinical/ clinical trial designs and executions
250+
Marketing clearances and approvals for IVD submissions
100+
Companion diagnostics regulatory filings in countries around the globe
Market Access and Commercialization Strategy & Support Experience
Trusted clinical research organization accreditations



