
Pharmacokinetic (PK) Assays
Precision advances the development of complex therapeutics including small peptides, antibody-drug conjugates (ADCs), PEGylated proteins, monoclonal and bispecific antibodies, and cell-based therapies like CAR-T, with a range of bioanalytical services.
To support PK studies at all phases of development, we offer assay development, validation, and implementation using ELISA and Meso Scale Discovery (MSD) assays for biologics, and flow cytometry and Droplet Digital PCR (ddPCR) for cell-based therapies.
Precision uses a variety of platforms for PK assays
- Molecular assays
- Immunoassay/Ligand-binding Assays
- Flow cytometry
-
Sensitive molecular assays for challenging drug modalities
Explore ddPCRFor newer drug modalities such as cell and gene therapies, Precision utilizes real-time polymerase chain reaction (also known as quantitative PCR or qPCR) and Droplet Digital™ PCR (ddPCR) to support discovery, preclinical, and clinical programs.

-
Immunoassays for any sensitivity or multiplex needs
Explore our immunoassay platformsDecide on required sensitivity, high sample throughput, and minimal sample volumes to choose the appropriate ligand binding assay for your biotherapeutic quantitation

-
Understand how your biotherapeutics are engaging targets with RO assays
Explore receptor occupancy assays by flow cytometryFlow cytometry is an accurate and effective method when used to identify and measure cellular biomarkers in complex subpopulations. As part of our bioanalytical labs, receptor occupancy assays measured via flow cytometry are a powerful tool for evaluating therapeutic engagement and thus efficacy.
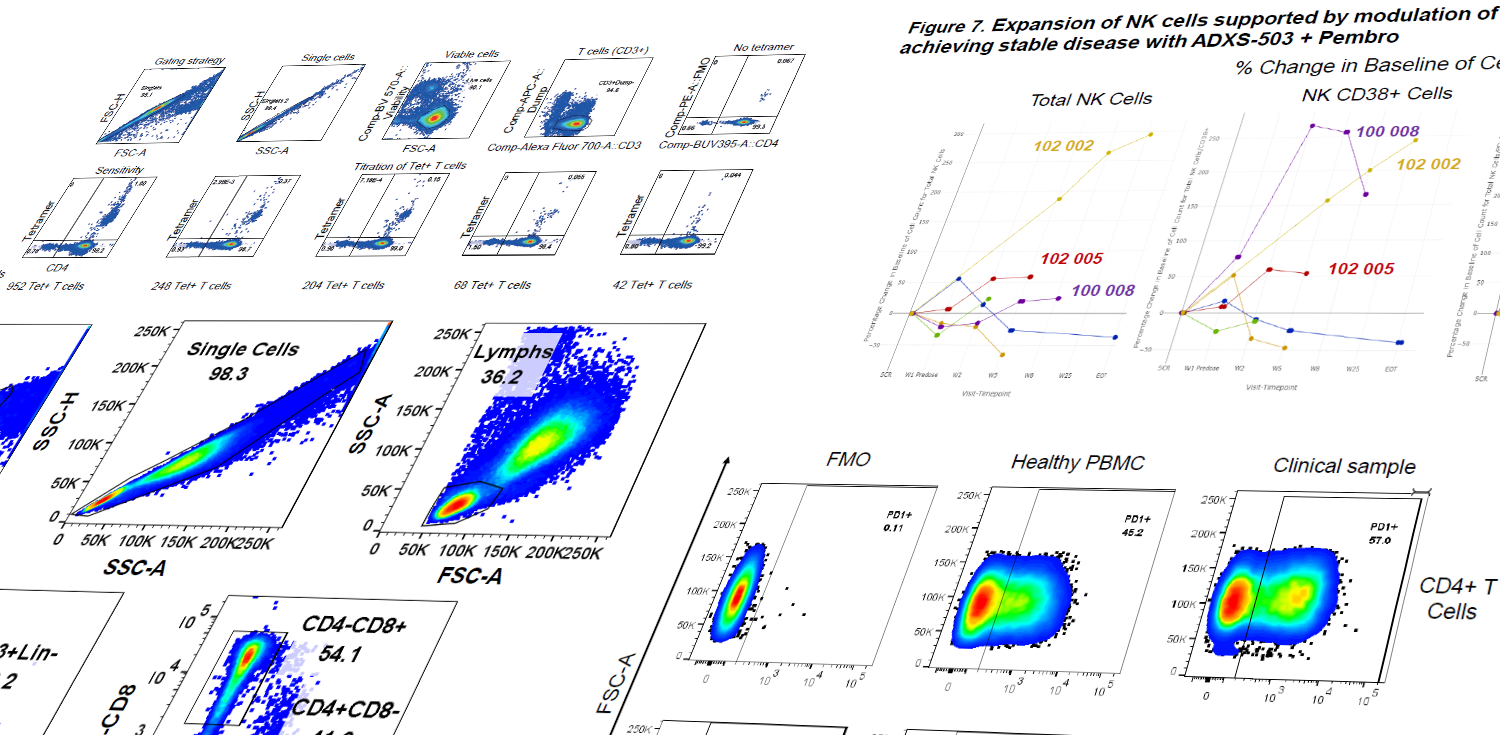
Study PK profiles of your unique cell and gene therapies
Precision's CGT team excels in developing PCR (ddPCR and qPCR) assays for a range of applications in cell and gene therapy. We routinely develop and validate custom PCR assays specific to client gene therapy vectors or cell therapies.


For gene therapy
- Biodistribution
- Shedding
- Common human matrices: saliva, urine, feces
- Transgene expression

For cell therapy
- CAR T cell (or other cell therapy) persistence/PK
- Replication-competent lentivirus (RCL) assay
Extensive experience with advanced platforms for PK analysis
We help you select the best platform for your project based on drug modality, required sensitivity, and regulatory guidance. We have extensive experience developing and validating assays to evaluate PK behavior throughout all phases of the drug development process using the following platforms:
Measure pharmacokinetics for any biologics and cell-based therapeutics
Define what you're studying
We offer assay development, validation, and implementation to obtain the following PK data:
- Absorption
- Cmax
- Bioavailability
- Half-life
- Clearance

Leverage end-to-end assay development support
Precision can address your assay development, validation, and testing needs from preclinical through pivotal trials.
- Unparalleled scientific leadership
- Fit-for-purpose – design, qualify, validate (GLP, GCLP, CLIA) the right assays at the right time
- Global scale – logistics to support global sample collection and storage in a 100,000 square foot state-of-the-art CAP-accredited and ISO-certified facility

Combine biomarker and clinical trial data
Precision has built a proprietary informatics platform, QuartzBio, to help researchers understand and interpret PK data in the context of other pharmacodynamic (PD)-related biomarker assay data, as well as in the context of key clinical trial annotations
Learn more about the computational, informatics, and sample management capabilities of QuartzBio.
Global reach, multi-site support
Our PK services support pre-clinical and clinical research, including multi-site studies, conducted anywhere in the world.

Consult with Precision for a personalized plan
Precision’s specialty lab scientists take a collaborative and consultative approach to projects and can provide recommendations on biomarker assay strategies and implementation. Services can be provided individually or as part of a comprehensive therapeutic development package including biomarker assays and clinical trials.
Related services
-
Explore

Tissue Cross-Reactivity
ExploreScreening biotherapeutics for tissue cross-reactivity (TCR) using tissue microarrays and providing full GLP TCR data as needed to support IND filing -
Explore
Immunogenicity
ExploreSpecializing in challenging immunogenicity projects, such as the evaluation of multi-domain proteins, PEGylated proteins, complement activation, and immunogenicity of CAR-T and gene therapies -
Explore

Large Molecule PK
ExploreDevelopment, validation, and implementation using ELISAs and MSD assays for biologics, flow cytometry, and ddPCR for cell-based therapies
