World-Class Expertise,
Logistics Drive Late
Phase Study
(IIb-III) Services
Built on a strong foundation of 375+ successfully completed Phase II/III clinical trials, which involved activating over 14,300 sites and enrolling over 78,000 patients, Precision understands the unique challenges of late phase studies period. We have developed solutions specifically to surmount the clinical, scientific, and logistical hurdles these studies face.
Some of these solutions are unique to Precision, like our proprietary Epiontis ID technology that makes immunophenotyping streamlined and cost-effective enough to use for even large, multi-site late phase studies. Many of these solutions involve our logistical and operational excellence, including world-wide medical monitoring as well as regulatory, CDISC, and eCTD services. All of these solutions are motivated by the singular goal of helping you bring your therapeutic to market as efficiently as possible.
Overcoming logistical challenges common to late phase studies
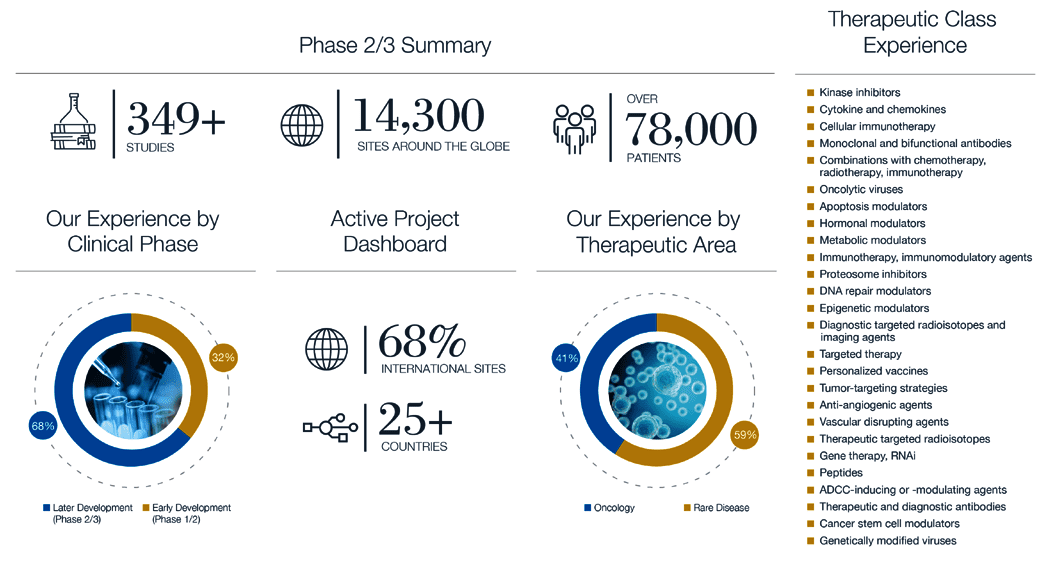
With the need for sufficiently large and diverse patient cohorts, late phase studies often take place at multiple sites that can be located in different countries. For sponsors running late phase biomarker-driven trials, managing the logistics around sample handling for biomarker analysis and coordinating the work of an outside specialty lab across sites and regions can be time-consuming and complicated. By working with Precision for late phase studies, you get all the support you need efficiently integrated into a single company.
Our global network of clinical study sites includes the world’s most respected organizations and is matched by a specialty lab footprint and clinical sample management processes that facilitates reliable and consistent generation of biomarker assay data. Our services cover everything from kitting and staff site training to ensure that standardized sample collection procedures are followed to global specimen transport and storage. And through our proprietary QuartzBio platform, you get a solution that provides virtual Sample inventory management so you always know where every sample is, as well as powerful computational and bioinformatics capabilities that harmonize clinical and biomarker data for real-time decision making.
Deploying biomarker assay technologies designed for late phase studies
Precision’s specialty labs provide the full range of technologies needed for late phase biomarker assays, including sensitive, high-throughput analyte measurement platforms like ELISAs, MesoScale Discovery (MSD) assays, and SIMOA® assays, and our proprietary Epionitis ID technology. Developed by Precision scientists as a cost-effective alternative to flow cytometry, Epiontis ID enables immunophenotyping at the scales needed by late phase studies and with the logistical constraints of global, multi-site trials.
Case Study: Exceeding enrollment expectations in a rescue study: A phase III registrational trial in multiple myeloma
Precision was identified by a sponsor to rescue global and US management of a phase III registrational trial in relapsed, refractory multiple myeloma patients. This study targeted the
enrollment of 780 patients at 155 sites in 20 countries. The sponsor’s primary motivation for pursuing the shift to Precision from the other contract research organization (CRO) was based on their recognition that clinical research associates (CRAs) with little to no multiple myeloma experience and only minimal monitoring experience had been placed on their study.
As a result of inefficiencies in operations, the study’s start-up was delayed.
Find out how Precision was able to quickly transition onto this study and complete the much-delayed patient enrollment one month early in the full case study.
Discover how our late phase study services can advance your clinical development program
Discover how our late phase study services can advance your clinical development program
Explore our services
Clinical Sample Management
Sample inventories from a global network of labs supply real-time processing in 55 countries; consolidated data from central labs, screening labs, and specialty labs with clinical data create actionable reports.
Clinical Trial Management
Fully integrated biomarker-driven trial management solutions-from study start-up through full-service execution-help accelerate your path to approval.
Epiontis ID: Molecular Phenotyping
Precision’s proprietary Epiontis ID technology delivers robust, repeatable, yet cost-effective immune cell phenotyping without the need for viable cells. With over 100,000 clinical samples analyzed, Epiontis ID is a globally-accepted immune monitoring technology.
