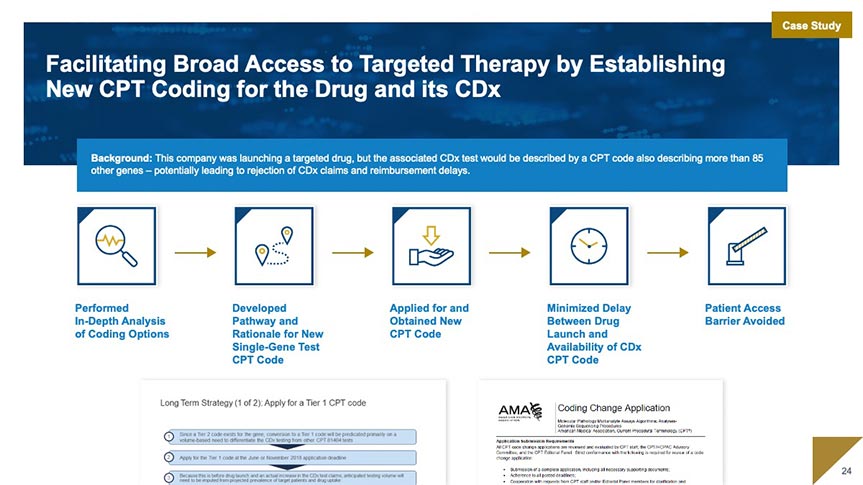
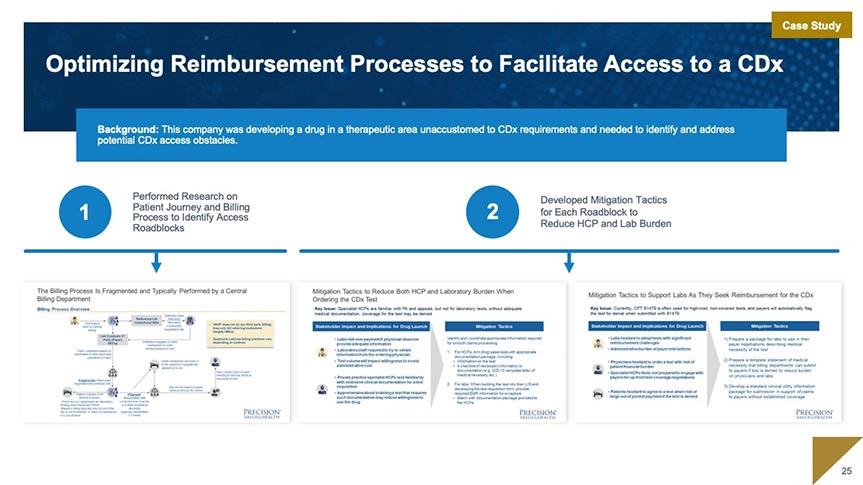
Regulatory & Quality
Solutions for LDTs,
IVDs, and CDx
Precision for Medicine provides support across the development spectrum, offering comprehensive regulatory planning and strategy, early agency interactions, design of analytical studies, and regulatory submissions. No matter where you are seeking approval, we can help you work with the FDA, EMA, MHRA, NMPA, and PMDA, and design a regulatory solution.
We can design regulatory solutions and facilitate global registrations for diagnostics and CDx in the United States, Canada, European Union, Ireland, United Kingdom, Norway, Iceland, Switzerland, Liechtenstein, Japan, China, and Australia among others.
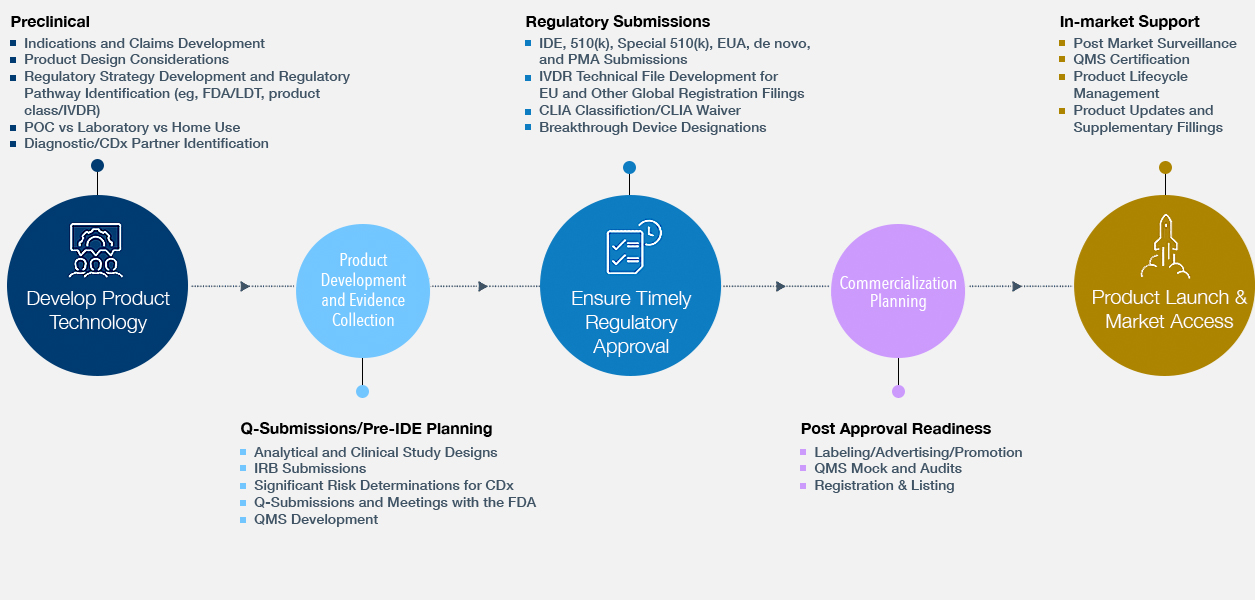
Accelerate Commercialization, From Bench to Bedside
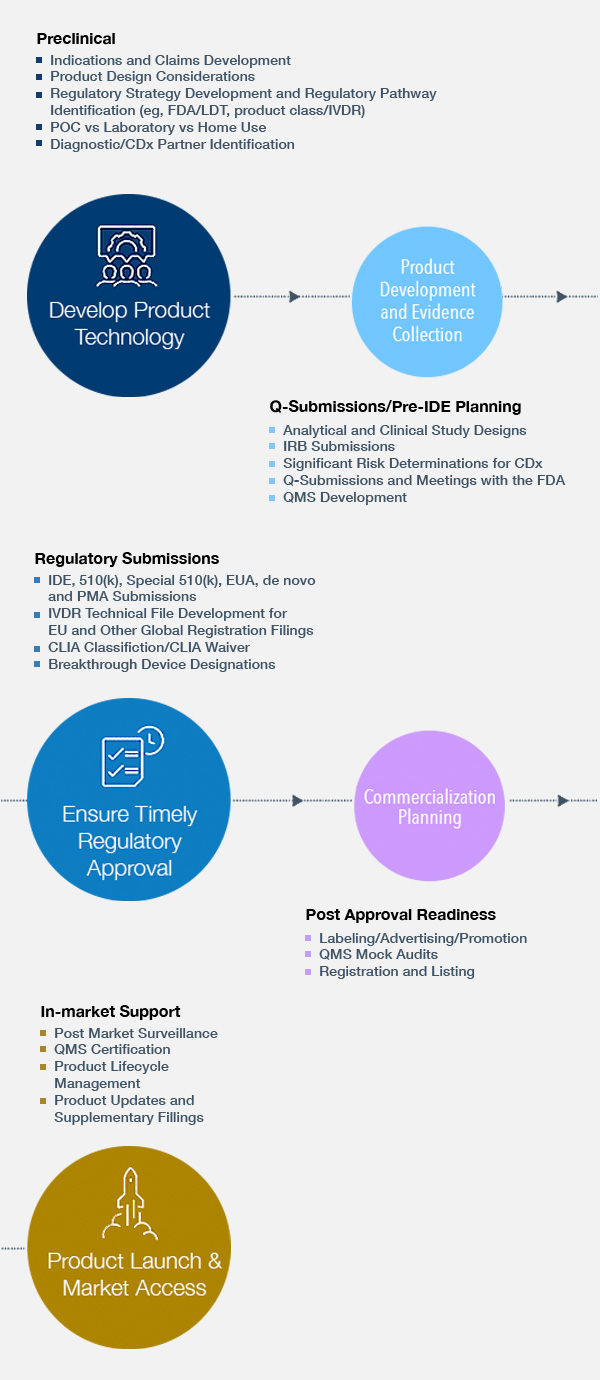
Regulatory Strategy & Submissions
- Preclinical Planning and Strategy: Product Design and Development Considerations
- Clinical and Analytical Studies: Design and Early Agency Interactions
- EUA/510(k)/De novo/PMA Submissions/IVDR Compliance and IDE
Regulatory Strategy & Submissions Experience


CDx Biomarkers for
Oncology Research & Development
Dr. Margaret Curnutte, Sr. Director, In Vitro Diagnostics & Quality
Advanced Biomarker Capabilities in Assay Development, Regulatory Strategy, Diagnostic Clinical Trials, Specimen Sourcing, Statistics, and Regulatory Submissions
Biospecimens to Support Your Diagnostic Development
![]()
Blood, Biofluids, and Derivatives
Diseased and healthy human blood, plasma, serum, CSF, stool, ascites fluid, saliva, urine, and more.
![]()
Tissues
Pathologist-verified, fresh, frozen, and fixed tissue specimens from healthy and diseased human subjects.
![]()
Liquid Biopsy
Comprehensive services including kitting, collection, processing, and profiling from your patients or ours.
![]()
Custom Biospecimen Collections
Global clinical network, regulatory approved, and ready to enroll.
![]()
Viable Cells
HLA-typed cellular products including PBMCs, BMMCs, Leukopaks, DTCs, and more.
Access Critical End-to-End Regulatory Support to Speed Your Diagnostic Product to Market
Services That Complement Regulatory Support Services: Biospecimens, Market Access, and Custom Assays
Biospecimens
Thousands of IRB-approved, clinically-annotated biospecimens ready to ship, same day, to your lab. Whether using specimens for assay development, bench research, or generating big data for your research or development studies, we deliver.
Market Access
Specializing in market access planning and strategy; coding, coverage, and payment; and evidence generation for coverage.
Custom Assays
Development of de novo assays with a particular specialization in cell-based assays and epigenetic immune cell phenotyping using our proprietary and patented Epionitis ID platform.