Precision
COVID-19
Resource Center
In these unprecedented times, we at Precision understand the critical need for time-sensitive solutions to address COVID‑19.
Precision supports your research with comprehensive resources that can be rapidly deployed to help ensure the conduct of your vaccine, therapeutic, or diagnostic development program.
COVID‑19 Clinical Development Solutions
Biospecimens to Support COVID‑19 Research
Diagnostics and IVD Development Support
COVID‑19 Clinical Development Solutions
Clinical Trial Design and Conduct
Full-service clinical trial execution with deep experience in respiratory and infectious diseases.
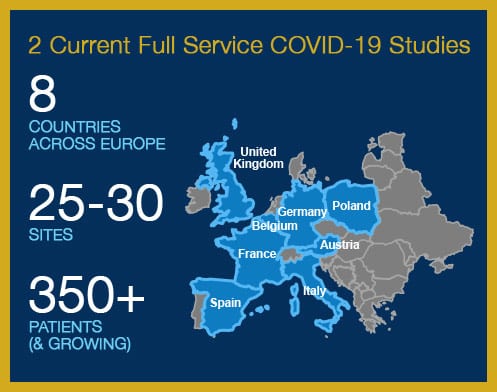
Precision is currently managing multiple COVID‑19 clinical trials.
Global Support for
30+
Respiratory and Infectious Disease Projects
- COVID‑19
- Pulmonary Arterial Hypertension
- Asthma
- Alpha-1 Antitrypsin Deficiency
- Adolescent Asthma
- COPD
- Cystic Fibrosis
- Respiratory Distress Syndrome; ABCA3 Deficiency
- Idiopathic Pulmonary Fibrosis
- Non-TB Bacterial Lung Infections
- Seasonal Allergic Rhinitis
- Influenza
- Hepatitis B
- Hepatitis C
- Hepatitis D
- HIV
- Malaria
Precision understands and can address the specific needs of COVID‑19 studies:
- Identification of site level processes and national/local guidelines to allow for rapid study start-up
- Knowledge of patient management and care guidelines to enable outreach to sites and physicians
- Verification of evolving EMA and FDA guidelines and a solution-oriented approach to obtaining patient consent, data capture, and data monitoring
Specialty Assays and Immune Monitoring Solutions for Any Development Stage
Immune status characterization is critical to assessing treatment efficacy.
Amidst public-private efforts, consistent assessment of immune status across studies may help compare treatment efficacy more effectively.
- Evolution of immune response to disease and clinical intervention
- Evaluate cytokine/chemokine storm
- Detect levels of 2019-nCoV in samples
- Quantify Tfh and Treg cells efficiently using Epiontis ID epigenetic immune cell phenotyping
- Full suite of FDA/EMA required assays to assess binding and neutralizing antibodies
Precision offers a suite of assays and immune monitoring technologies to support any stage of COVID‑19 vaccine or therapeutic development.
- Flow cytometry – 18 color
- Epiontis ID – 31 validated assays
- Mesoscale Discovery (MSD)
- Quanterix SIMOA
- Luminex
- ELISA
- ELISpot
- Fluorospot
- RT-qPCR and ddPCR
- Nanostring
- Mesoscale Discovery (MSD)
- ELISA
- Biacore
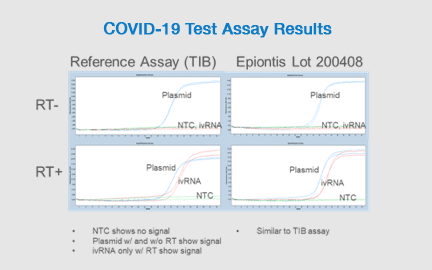
SARS-CoV-2 Infection Testing
Precision has developed and validated a test for COVID-19 conforming to WHO standards.
Key Benefits of the Precision Assay:
- Tightly controlled: Automated measurements to decrease variability
- High-throughput: Up to 400 samples per day
- Can be performed in parallel with immune monitoring of clinical samples
Novel Immune Monitoring Solution to Address Logistical and Time Challenges: Epiontis ID
- Proprietary epigenetic-based technology
- Strong correlation with flow cytometry
- Science and quality established with leading organizations – Roche/Genentech, BMS/Celgene, GSK, Lilly
- Assay can be run on a variety of sample types
- Home monitoring possible via dried blood spot cards
- Intact cells not required – supports logistical disruptions
- High throughput and scalable
- Precise measurements of Tfh and Treg cells: emerging markers of disease progression

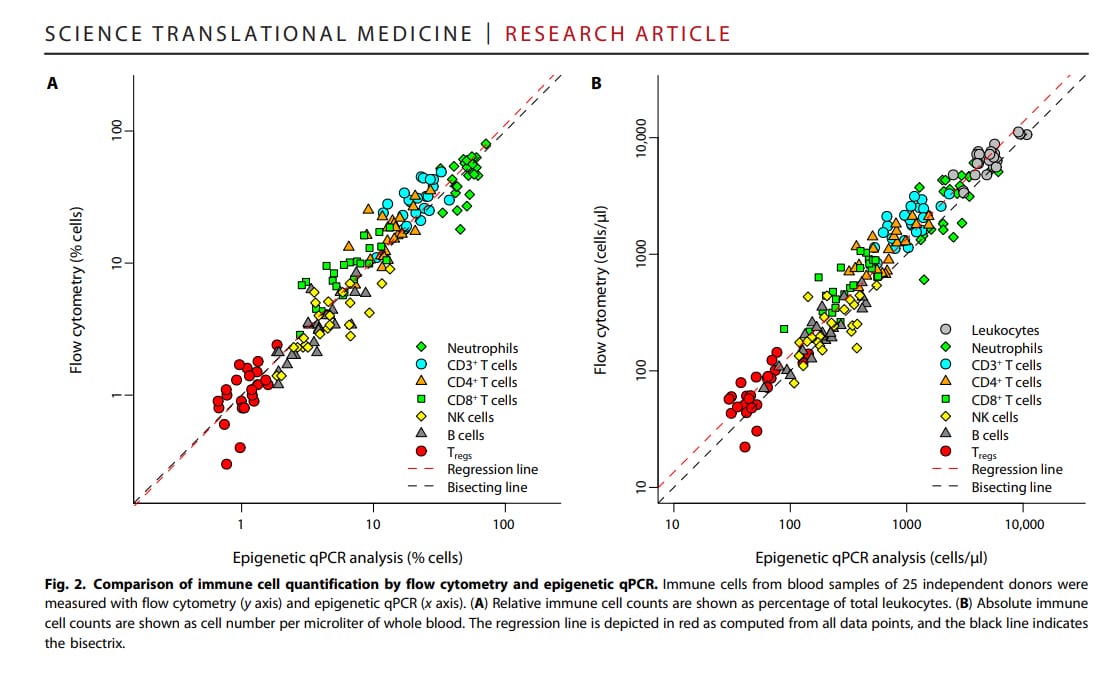
Epiontis ID works with samples once considered non-viable and delivers results comparable with traditional flow cytometry.
Epiontis ID Panels for COVID‑19 Specific Applications
SARS-CoV2 Trials
- Overall T cells
- CD4 cells
- CD8 cells
- CXCR3+ cells
- Tfh cells
- Overall B cells
- Memory B cells
Anti–IL-6 R Trials
- Neutrophil granulocytes
- CD56 dim NK cells
- Overall B cells
- Memory B cells
- Tregs
- CD4 cells
- CD8 cells
Antiviral Trials
- Tregs
- T fillicular helper cells
- CD4 cells
- CD8 cells
- Eosinophils
- Neutrophils
- CD56 dim NK cells
- Overall B cells
- Memory B cells
Global Sample Logistics and
Sample Processing
- Worldwide Processing Lab network and 6 Precision Specialty Labs in US and EU; all sites continuing operations
- Processing of any fresh tissue to any format needed for assays (PBMCs, nucleic acids, slide prep)
- Long-term data on globally consistent viability and recovery; >80% viability over 10 years
- GLP (preclinical) and GCLP (clinical), ISO 13485:2003 and ISO 17025:2017; CAP, CLIA
- 100,000 square foot facility including 25M sample storage infrastructure
- Biostorage avaialble for COVID‑19 samples


In-House Capabilities to Work With All Sample Types
Virtual Sample Inventory Management (vSIM)
Site pressures, remote monitoring, and potential for disruptions highlight the need for an ongoing view into sample status.
- Ongoing visibility into sample status is key to detect and address issues critical to sample integrity
- Crucial in cases of alterations to sample processing, storage, or analysis facilities
Precision’s QuartzBio vSIM solution:
- Consolidates sample inventories within a clinical trial to provide ongoing, comprehensive visibility into samples to identify site-specific and other potential quality issues
- Provides forward-looking visibility into expected sample collection; enables coordination with labs
- Captures information FDA and EMA are requiring for documentation of COVID‑19 impacts
Translational Informatics and Computational Biology
Harness public data and domain expertise to accelerate your COVID‑19 research.
The QuartzBio team transforms multiomic data into insights, spanning discovery-oriented preclinical work to regulated clinical trials. We work with teams to use AI and machine learning (ML) approaches specifically developed to work with clinical and biomarker data-all guided by domain experts with real-world biological understanding. It is this unique and tailored computational biology approach that allows QuartzBio to derive the insights that support and accelerate development programs.
- Perform target pathway selection
- Identify repurposing opportunities
- Characterize mechanism of action (MoA)
- Stratify patients
- Provide submission-ready results to support regulatory filings
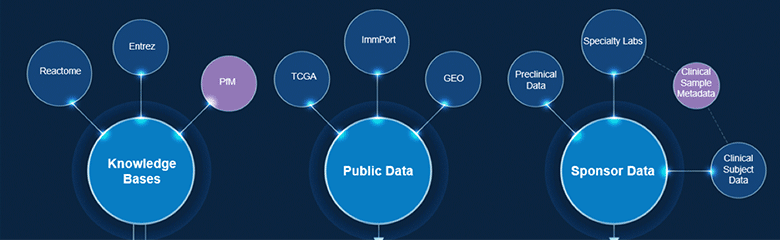
COVID-19 Data Aggregation Initiative
Publicly available data increasingly represent an asset to efficiently generate and test hypotheses with data-driven and scientifically informed approaches.
Applications include repurposing candidates for target pathways and identifying combination therapies. Our team is undertaking the creation of a unified SARS-CoV-2 / COVID-19 data ecosystem as a key tool for the scientific community to use in this once-in-a-generation fight.

Biospecimens Solutions for COVID‑19
Application, Assay, Vaccine, and Therapeutic Development
EUA – Sample Sets
Available Immediately – Prepared in accordance with FDA guidance
Swabs and blood specimens:
- Molecular
- SARS-CoV-2 Positive (n=30)
- SARS-CoV-2 Negative (n=80)
- Serological
- IgG/IgM: SARS-CoV-2 Positive (n=30)
- IgG/IgM: SARS-CoV-2 Negative (n=80, of which 10 must be HIV+)
Characterized by EUA-Approved Assays
Patient Specimens (non-contrived)
Assay Development 510(k) Preparation
Remnant Specimens from our CAP-/CLIA-certified global labs
Molecular Assays
- Naso and oral swabs: SARS-CoV-2 RNA
- Verified EUA method and results
Antibody Assays
- Serum and plasma: SARS-CoV-2 IgG/IgM
- Clinical Specimens from our clinics and research units
- COVID-19 diagnosed (confirmed)
- Matched sets
- Days 0, 4-11, 12-21, 21+
Contract – Research Collections
Research Collections – Samples to support the screening, diagnosis, and monitoring of COVID-19
- Convalescent plasma
- Leukopaks
- PBMCs
- Serum/Plasma
- Saliva/Sputum
- Swabs
Custom Collections
- Point-of-care devices
- Capillary/dry blood spots
- Matched sets
COVID-19 Research Registry
- ACUTE patients
- RECOVERED patients
✅ IRB approved/Informed consent
Precision Biospecimens
25
Million Biospecimens Managed
55+
Countries with Real-time Sample Processing
500+
Diagnostic & Therapeutic Trials Conducted
IVDs and Diagnostics for COVID‑19
Regulatory and Developmental Consulting
For diagnostics companies developing:
- Point-of-care tests from multiple sample sources (Precision can supply biospecimens including nasopharyngeal swabs, blood, saliva)
- IgG, IgM, and IgA tests
- Home collection and/or home use tests
- Alternative COVID-19 responses including non-COVID-19 diagnostics applicable to COVID-19 patients
As well as laboratories bringing in their own or an approved EUA test.
Precision’s IVD and Diagnostic Expertise
100+
IVD presubmissions, preclinical/clinical trial designs, and executions
250+
marketing clearances and approvals for IVD submissions
100
Dx regulatory filings in countries around the globe
Family-Friendly Resources
Today we are dealing with unprecedented circumstances. Now more than ever, Precision’s guiding principles of Client Service, Purpose, Accountability, Mutual Respect, and Collaboration serve as the foundation for continuing to support advances in therapeutic innovations worldwide.
The reality is that we cannot continue to do this important work without the backbone of these efforts-our people. The WHO and many other
authorities stress the importance of protecting mental health, and have offered foundational advice to “be empathetic.” Like you, we are taking many steps to support our teams, and we have had an overwhelming show of appreciation for a simple list of resources and activities for families adjusting to the new work from home realities. We invite you to visit our password-free microsite for access to these family-friendly resources.


