Clinical Data Management Services
Meticulously detailed and flexibly delivered, Precision’s clinical data management services team delivers data you can trust using the electronic data capture (EDC) system that works for your study. Working hand-in-hand with you and study site personnel, our reliable team ensures you extract maximum insight from your clinical trial data.
An Experienced Team of Clinical Data Management Professionals
Ensuring data integrity from start-up to submission
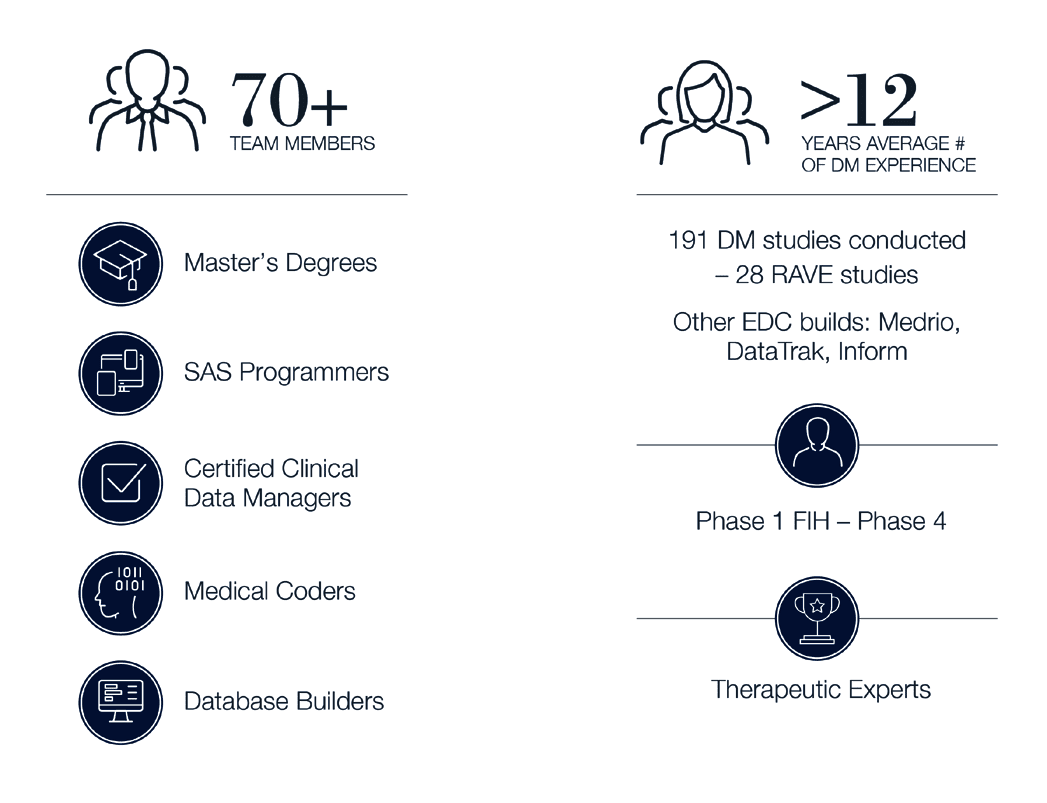
Choose a clinical data management team with Precision
Clinical Data Management (CDM) is vital to properly perform clinical research. When executing data collection, Precision experts build data that is credible and comprehensive to drive an expedited process between drug development, approval, and market placement.
We understand that at the end of a trial, it’s all about the data. Starting with the creation of your custom clinical data management plan, through the database lock stage you’ll find Precision to be meticulous in every stage.
End-to-end coverage for your clinical data management needs
The same reliable clinical trial data management team with indication-specific expertise supports your project every step of the way. Our system features:
Clinical database management start-up
- Develop custom clinical trial data management plan based on your protocol, study site needs, and your needs
- Use best EDC for your needs
- Design CRFs and write CRF guidelines
- Coordinate data-in
Clinical database development
- Build and screen the database, including testing and QC
- Coordinate user acceptance training (UAT) with sponsor and study site
User administration
- Set up accounts
- Conduct training
Clinical database conduct
- Data Cleaning
- Query Resolution
- Medical Coding
- External Vendor Data Transfers & Reconciliation
- SAE Reconciliation
- Ongoing EDC Metrics, Clean Submit Report
- ‘SMART’ Patient Profiles
Clinical database lock
- Obtain PI signature
- Lock CRFs
- Generate final datasets
- Site CRF Archival CDs
- Decommission EDC
Case Study: Securing FDA approval for a new diagnostic when compared to the “gold standard”
In diagnostic trials, it is common and often expected to compare performance of a new diagnostic to the “gold standard” based on current standard of care. After planning a study to assess typical measures of sensitivity and specificity, our sponsor was asked by the FDA to modify the primary endpoint used to interpret whether the new diagnostic was sufficiently comparable to the existing gold standard. The sponsor turned to Precision for Medicine to navigate the request.
Tell us about your clinical trial data management needs
Integrity matters, whether it’s describing your study data or the teams generating and managing your study data. Ensure integrity with Precision.
Tell us about your clinical trial data management needs
Integrity matters, whether it’s describing your study data or the teams generating and managing your clinical trial data. Ensure integrity with Precision.
Precision Clinical Trial Services
Global Clinical Trial Footprint
Sample processing labs, clinical trial sites and offices in five continents provide the clinical reach and scale to manage complex global programs.
Clinical Development Strategy
Tailored strategies consider the scientific, regulatory, and commercial factors that shape each trial, mitigating risk and advancing the development pathway.
Clinical Trial Design
Advanced trial-design approaches—including basket, umbrella, and adaptive trials—deliver biomarker driven clinical research. Deep experience in these highly complex trial designs maximizes both insights and efficiency.
Biostatistics
Seasoned biostatisticians and statistical programmers deliver insight into every trial phase, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Clinical Sample Management
Sample inventories from a global network of labs supply real-time processing in 55 countries; consolidated data from central labs, screening labs, and specialty labs with clinical data create actionable reports.