Specialized
Therapeutic Area
Expertise to Accelerate
Clinical Development
Innovative therapeutics and diagnostics change the way we ensure health and manage disease. To effectively bring these products to market, the clinical development process must progress as well. Targeted therapies, companion diagnostics, cell and gene therapies, these novel treatment modalities require equally novel approaches when it comes to evaluating safety and efficacy. This is why successful development requires a partner who is not only experienced, but has the scientific, clinical, and logistical acumen to build creative solutions to new challenges. It’s also why we formed Precision.
By bringing these distinct services together into a single global organization, we create opportunities for insight into patient biology that are simply unachievable when these activities are conducted independently. While recognized as a worldwide leader in oncology, immuno-oncology and rare disease clinical trials, our expertise spans numerous therapeutic areas and stages of development.
Globally recognized therapeutic areas of expertise
Oncology & Immuno-Oncology
Through 20+ years running successful oncology trials, we’ve developed a unique blend of proprietary technologies, flexible processes, and creative problem solving abilities that advance even the most challenging clinical development programs.
Rare & Orphan Disease
With experience built on 150+ orphan disease projects covering 80+ rare diseases, we know how to anticipate logistical and regulatory obstacles and craft bold solutions that drive rare disease development programs.
Cell & Gene Therapy
With team members who are global thought leaders in cell and gene therapy development, Precision brings together scientific, clinical and regulatory expertise that is advancing the development of these programs as well as oncolytic viruses, cancer vaccines and radiolabeled ligands.
Rapidly expanding therapeutic areas of expertise
Neurology
We combine advances in neurology biomarkers and biomarker detection technology for robust therapeutic development.
Autoimmune & Allergy
We support development programs with state-of-the-art immune monitoring solutions and immunogenicity testing expertise.
COVID-19
We are engaged in COVID-19 research with comprehensive solutions for all areas of therapeutic, vaccine, and diagnostic development.
Pediatrics
Advanced clinical trial design expertise and sensitive biomarker detection technologies accelerate pediatric therapeutic development.
Companion Diagnostics
Our integrated clinical development, biomarker assay, regulatory strategy, and commercialization capabilities deliver unique value for CDx programs.
Research phase expertise
Preclinical Development
Driving IND-enabling studies with comprehensive biomarker detection technology, our QuartzBio computational and informatics platform, and regulatory expertise.
Early Phase Trials
Integrated clinical and biomarker assay expertise, especially in advanced, biomarker-driven clinical trial designs, enables efficient and robust clinical studies.
Late Phase Trials
Experienced clinical trial operations, regulatory, and biostatistics teams integrate with proprietary late-phase biomarker technologies to advance therapeutic development.
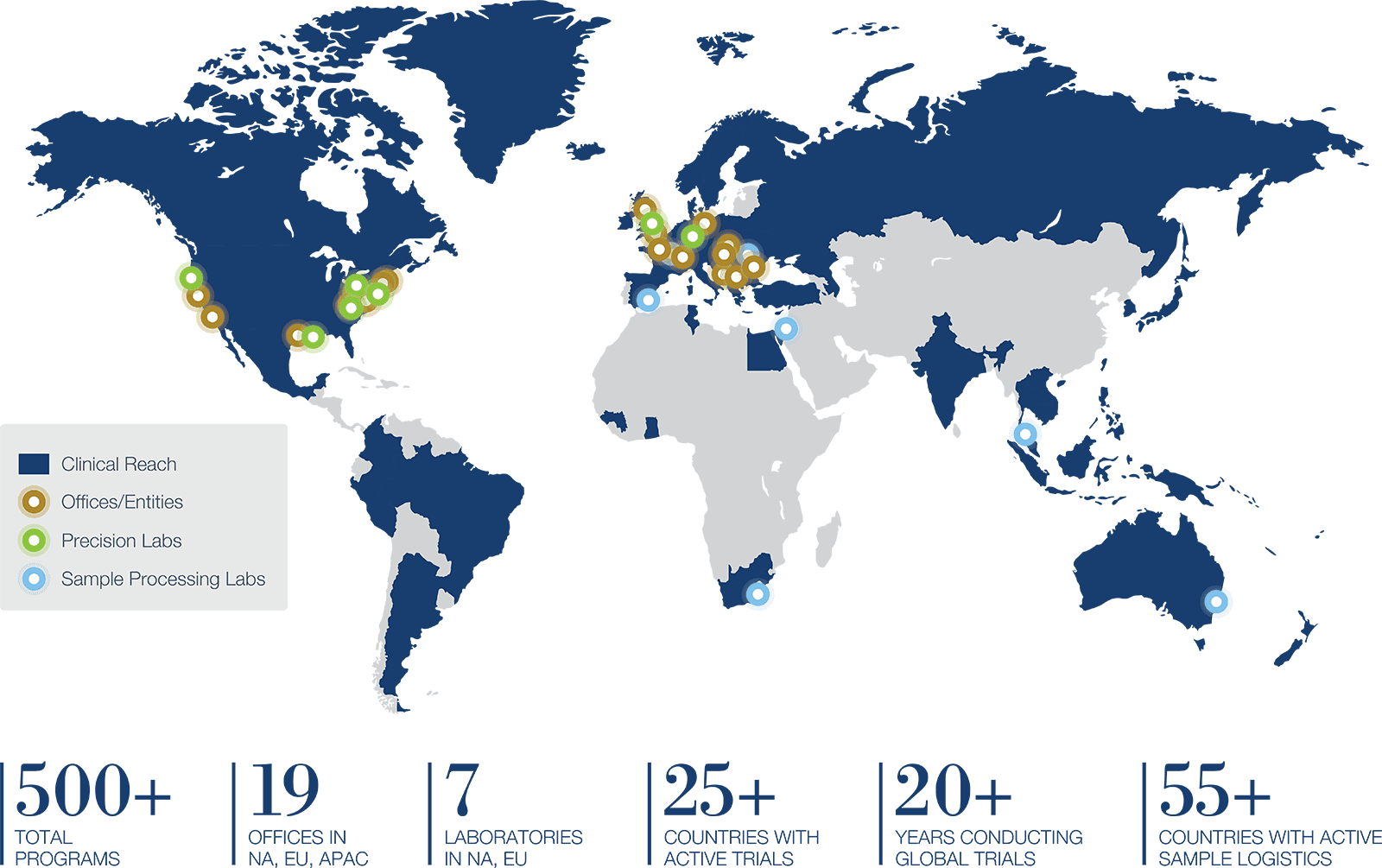
The scale to manage every aspect of complex global clinical trials
Trial services, sample processing and logistics, specialty labs