.jpg?width=1440&height=672&name=decentralized-clinical-trials%20(1).jpg)

Decentralized Clinical Trials
As the global pandemic has made site visits increasingly difficult, decentralized clinical trials (DCTs) have provided a solution.
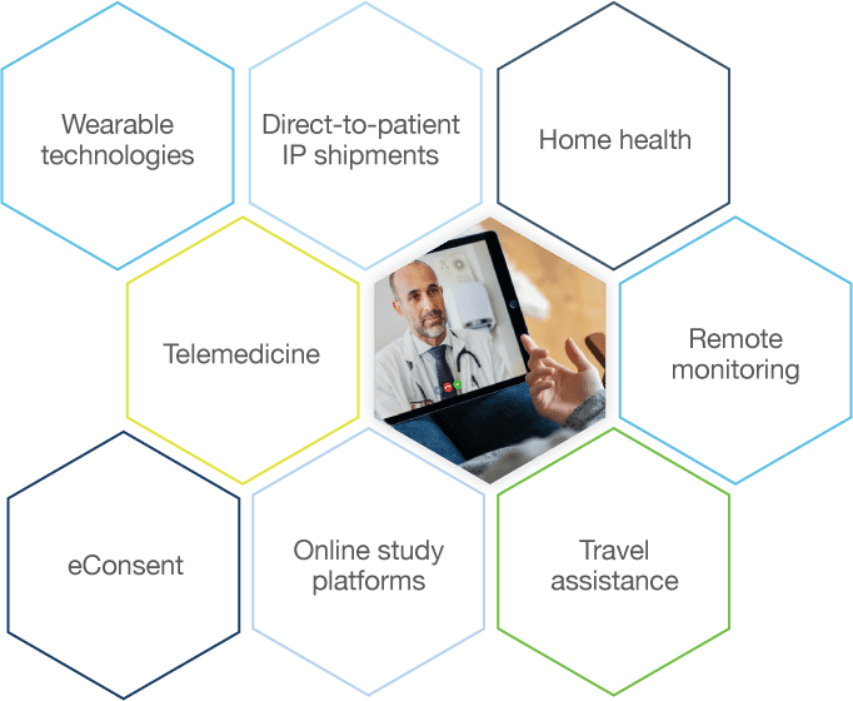
Count on Precision expertise honed over decades running virtual and hybrid studies. We harness a range of options—tailored to the needs of each trial and patient—including electronic data collection, telemedicine, and home health nurses. Whether enrolling patients, launching studies, or collecting safety and efficacy data, we deploy critical skills that streamline processes and reduce the patient burden. That’s good for every trial.
A range of DCT services and capabilities customized to your trial needs
30+ specialist vendors partnering with Precision around the world
to ensure the success of your decentralized trial
to ensure the success of your decentralized trial

DCT experts since 2014
We’ve conducted trials for over 80 diseases, all over the world. This experience has led to a full portfolio of well-honed systems, processes, and relationships to support decentralized clinical trials. Lessening the burden on the patient has always been a mantra for Precision. We can meet the new demands the pandemic has placed on trials.
Patient-centric trials start at home
- Digital technologies collect safety and efficacy data wherever the patient is
- Umbrella trials
- Patients for a single study can span the globe
Fewer clinic visits reduce patient/caregiver burden
Deep experience with debilitating disease and unique modalities
Like you, we understand that there’s a patient throughout the development pathway. Supporting our partners to bring innovative treatments to patients is our purpose. Leverage our experience in these therapeutic areas.
Tell us about your project requirements
Our expert team is ready to support your decentralized clinical trial needs, ensuring maximum flexibility and patient engagement. Start the conversation today.









