Flow Cytometry Analysis and Testing Services
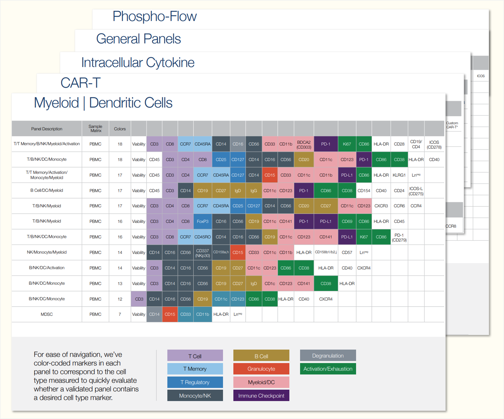
Our scientists develop complex multicolor panels, supporting sponsors based on context of use from exploratory, secondary, primary, and CLIA flow assays.
We routinely run complex assays, including receptor occupancy, CAR and cell therapy expression/function, antigen-specific flow, and phospho-flow. One core focus and area of expertise within our flow cytometry services is immunophenotyping. This is a crucial part of our comprehensive immune monitoring solutions, which are essential for multi-site clinical studies. These studies demand rigorous immunophenotypic analysis, particularly in trials for novel immunotherapies or cell therapies.
Measure endpoints for a full range of applications
All assays are customizable and may be developed according to ISO/GCP/GLP/CAP/CLIA regulations.
-
Rare cell detection and quantification based on phenotype, apoptosis, or phosphorylation status
-
Immunophenotyping and absolute counting of cell subsets
-
Modulation of activation and exhaustion markers
-
Apoptosis and cell cycle analysis via histone methylation
- Cytokine production
For CAR NK-cell/T-cell therapy:
- Expression of lineage-specific markers and characterization
- CAR T/NK persistence and immunogenicity
Approaches to leverage flow cytometry
- Receptor Occupancy (RO)
- Real-Time Flow
- Prevalidated & Custom Panels
- Flow Cytometry in Cell Therapy
-
Receptor Occupancy (RO)
Explore our receptor occupancy assay capabilitiesUnderstand how biotherapeutics engage their targets
- Simultaneous detection of RO and other surface markers using multiparametric flow cytometry
- Reagent specificity and sensitivity determined by extensive characterization during assay development
- Optimal matrix selection: eg, whole blood or fixed whole blood

- Simultaneous detection of RO and other surface markers using multiparametric flow cytometry
-
Real-Time Flow
Support clinical studies with whole blood and tissue flow analysis of clinical samples using custom or prevalidated panels
- Sample tracking and accession with same-day/next-day flow sample processing
- Global flow cytometry centers of excellence to support exploratory to GxP/CLIA sample processing
- Rapid and accurate flow analysis by our flow experts
- Assays are validated for intended use suitable for proof of concept, exploratory, secondary, or primary clinical trial endpoints.

- Sample tracking and accession with same-day/next-day flow sample processing
-
Prevalidated & Custom Panels
Expedite clinical development with “off-the-shelf” panelsView our prevalidated flow cytometry panels
- Validated fit-for-purpose flow assays using industry standards
- Add additional markers to any panel to address key PD markers for your clinical study
- Generate robust and reproducible data for clinical trials, and generate data for patient selection
- Complex assays measuring antigen and cellular specific responses using tetramers, target specific peptides, and CAR-T/CAR-NK flow

- Validated fit-for-purpose flow assays using industry standards
-
Flow Cytometry in Cell Therapy
Explore our flow cytometry capabilities for cell therapyMonitor the persistence and differentiation of cell therapy products
- Evaluate CAR expression on therapeutic cells
- Measure CAR T or CAR NK cell persistence and therapeutic effect
- Assess cell functionality and potency of immune system response to cell therapy/CAR

- Evaluate CAR expression on therapeutic cells

Leverage Precision's expertise across flow cytometry assays
Precision routinely runs complex and custom assays for clinical trials under rigorous quality control procedures.
-
Intracellular cytokine secretion (ICS)
-
Receptor occupancy (RO) assays and ligand measurement
-
Phospho-flow for phosphorylation state
-
Immune functional assays: antibody-dependent cell-mediated cytotoxicity (ADCC), T-cell activation
-
Antigen-specific responses: detection and characterization of peptides, dextramers, tetramers
-
Cytokine measurement using cytometric bead assays
-
Histone methylation measurement
-
Spectral flow cytometry
Target the clinical biomarkers important in your trials
Our panels are carefully designed and curated, incorporating a trial-specific approach that combines expert input with years of flow cytometry experience. Customization is available.
- Evaluate investigational agents in preclinical and clinical studies on a multi-site and worldwide basis
- Perform biomarker discovery by identifying patient populations most likely to respond to therapy and monitoring pharmacodynamic changes on target cells in response to treatment
- Customized sample collection kits and logistical services ensure optimal sample integrity
Precision's range of flow cytometry instruments and capabilities can accommodate any project
 BD FACSCanto™ II BD FACSCanto™ II |  BD FACSAria™ Fusion BD FACSAria™ Fusion |  BD LSRFortessa™ BD LSRFortessa™ |  BD FACSymphony™ A5 BD FACSymphony™ A5 | .png?width=237&height=220&name=Cytek%20Aurora%20(resized).png) Cytek Aurora Cytek Aurora |
|
|---|---|---|---|---|---|
| Para-meter | 10 | 18 | 20 | 31 | 64 |
| Colors | Up to 8 | Up to 16 | Up to 18 | Up to 30 | Up to 40 |
| Lasers | Up to 3 | Up to 5 | Up to 5 | Up to 9 | Up to 5 |
| CLIA Assays | Validated CLIA assays |
Start with the appropriate sample type for your flow cytometry project
Consult with Precision to address the unique needs and challenges of your clinical trial, starting with sample choice, to ensure robust, reproducible flow cytometry data.
| Flow Cytometry Applications | Untreated Blood | PBMC | Fixed Blood |
|---|---|---|---|
Immunophenotyping/Activation/Anergy |
✓ | ✓ | ✓ |
Vaccines/Antigen-Specific Tetramer |
✓ | ✓ | |
Peptide Flow/Intracellular Cytokine Secretion (ICS) |
✓ | ✓ | |
Cell Therapy Flow (CAR T, CAR NK) |
✓ | ✓ | ✓ |
PK Flow |
✓ | ✓ | |
Receptor Occupancy |
✓ | ✓ | |
Single Cell Sorting |
✓ | ✓ | ✓ |
Intracellular/Intranuclear Staining |
✓ | ✓ | ✓ |
Phospho-flow |
✓ | ✓ |
.jpg?width=2000&name=iStock-171579293%20(1).jpg)
Ensure sample quality with global sample processing and specimen logistics
We use standardized processes that follow each sample from collection to analysis and our biorepositories can store fluid and solid biological specimens at controlled ambient temperatures, +4°C, -20°C, -80°C, and in liquid nitrogen.
-
Real-time inventory tracking via e-Portal dashboard
-
Global PBMC processing same day or next day
-
Regular competency assessment and monitoring to ensure site-to-site and lab-to-lab data comparability
-
A biorepository with redundant back-up systems, dedicated back-up units and other safety and security features
Proven Results
Explore how researchers use Precision flow cytometry to power impactful science.
Explore more resources-
Poster
Validation of a Whole Blood and Proteomic Stabilized Blood Assay to Monitor the Engagement and Modulation of CD6 on T cells by Itolizumab as a Clinical Pharmacodynamic
Download Poster
-
Poster
An Assay to Monitor the Engagement and Modulation of CD6 on T cells as a Clinical Biomarker of Treatment with Itolizumab
Download Poster
-
Poster
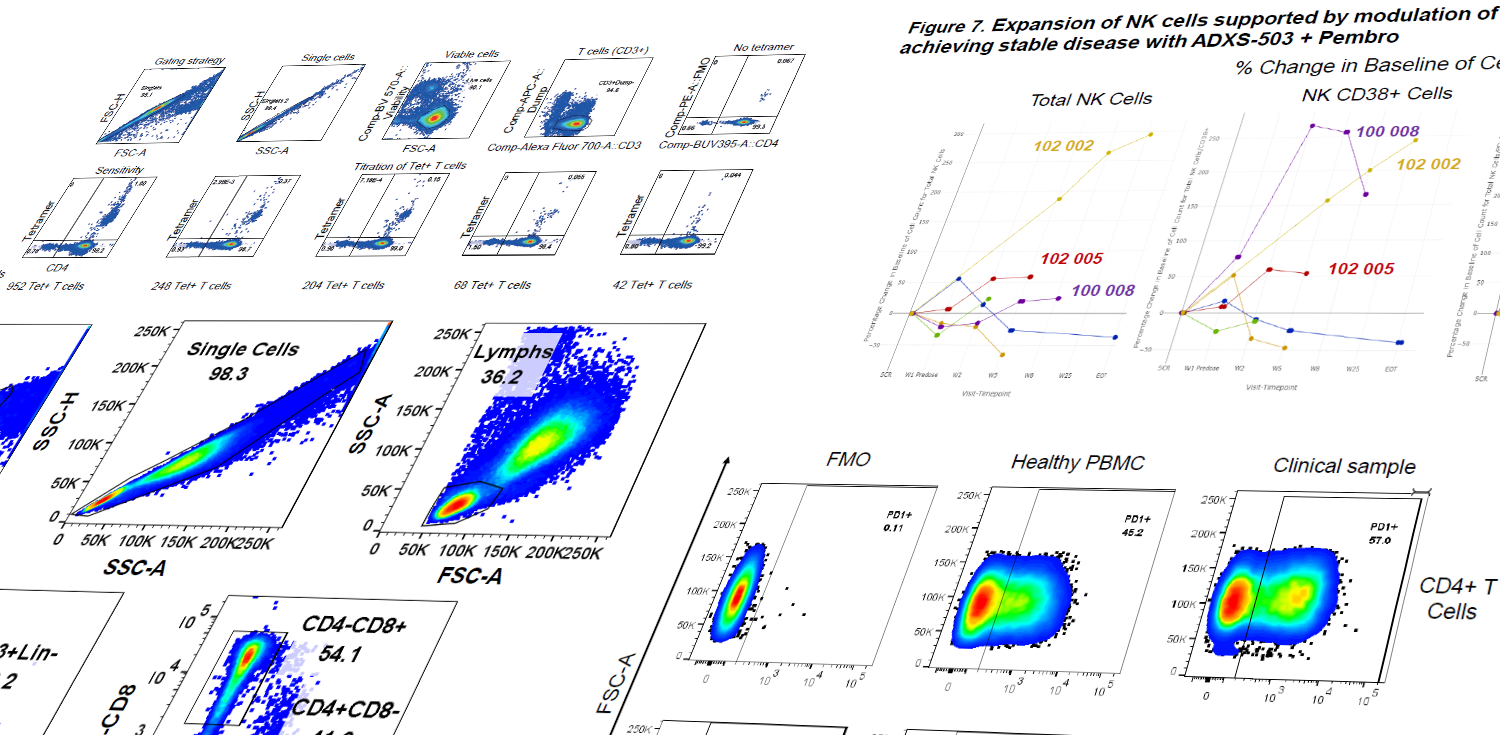
Evaluation of total PD-1 expression using multi-color flow cytometry in Metastatic Non-Small Cell Lung Cancer patients treated with Multi-Neoantigen Vector (ADXS-503) in
Download Poster%20in.jpg?width=252&height=245&name=93.%20Evaluation%20of%20total%20PD-1%20expression%20using%20multi-color%20flow%20cytometry%20in%20Metastatic%20Non-Small%20Cell%20Lung%20Cancer%20patients%20treated%20with%20Multi-Neoantigen%20Vector%20(ADXS-503)%20in.jpg)
-
Poster
Development and technical validation of tetramer staining for use as a biomarker for assessing gluten-specific T cells in clinical studies
Download Poster
-
Poster
Validation of BTK & Aiolos Immunophenotyping Assay to Monitor B, NK & T cell Responses as a Clinical Biomarker in B-cell Malignancies
Download Poster
Values of Precision flow cytometry
-
Established experience
Established experience running complex flow cytometry in gene therapy, cell therapy, immuno-oncology therapies, validating up to CLIA -
Senior staff
Sample testing by experienced staff who understand the disease biology and gating strategy -
Quality control
Real-time Quality Control during the run to ensure sample integrity

Conduct trials globally with multi-site support for flow cytometry assays and analysis
We provide flow cytometry services that support preclinical and clinical research, including multi-site studies, conducted anywhere in the world. Samples are processed same day or next day and utilize proven SOPs to ensure sample integrity.
Collaborate with us for a personalized plan
Precision’s flow cytometry service scientists take a collaborative and consultative approach to projects and can provide recommendations on assay strategies and implementation, if needed. Services can be provided individually or as part of a comprehensive therapeutic development package including biomarker assays and clinical trials.