
Autoimmune Therapeutic Development Services
Advanced biomarker capabilities designed to meet the needs of autoimmune studies




.jpeg?width=343&height=396&name=AdobeStock_119321858%20(1).jpeg)
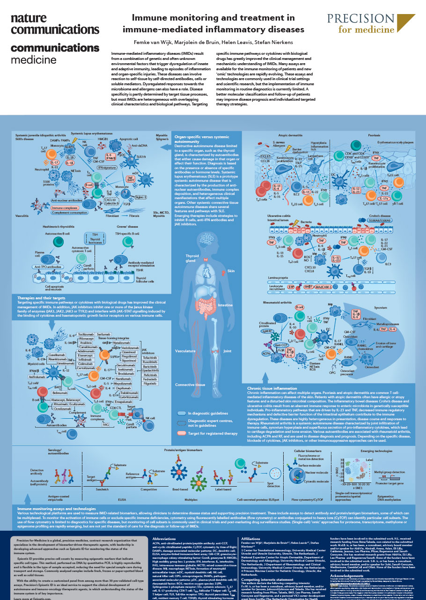
Precision has expertise in autoimmune disorders and their cellular pathways
Created in collaboration with Nature, this poster maps out the numerous cellular pathways relevant to many autoimmune disorders, as well as common treatments and the cellular targets of those treatments.
Precision’s cell phenotyping technology, Epiontis ID, is ideal for use in autoimmune studies
Epiontis ID has been extensively used for immune monitoring in autoimmune studies across a range of indications, and has validated assays designed for specific autoimmune indications.
Below is data showing the number of studies Epiontis ID has been used for in various autoimmune indications.
| Indication | Number of studies | Number of samples | Study phase | Study years | Sample types |
|---|---|---|---|---|---|
Crohn's Disease |
4 |
9468 |
Phase 3 |
2018 |
Blood |
GvHD |
5 |
3411 |
Phase 3, research |
2016, 2017, 2018, 2019 |
Blood, tissue, PBMC, cells |
Psoriasis |
6 |
2834 |
Phase 1, 2, 4 |
2015, 2016, 2017, 2018 |
Blood, tissue |
Ulcerative Colitis |
2 |
1575 |
Phase 2 |
2015, 2016 |
Blood, tissue |
Asthma |
1 |
1350 |
Phase 2b |
2018 |
Blood |
Lupus |
2 |
1312 |
Phase 1, 2 |
2017, 2018 |
Blood |
Behcet's Syndrome |
1 |
672 |
Phase 4 |
2015 |
Blood |
Sjorgren's Syndrome |
5 |
479 |
Phase 1, 2a |
2015 |
Blood, DNA, PBMC |
Crohn's, Multiple Sclerosis/ Ulcerative Colitis |
2 |
390 |
Phase 1 |
2018 |
Blood |
Rheumatoid Arthritis |
2 |
375 |
Phase 1b, 2 |
2014 |
Blood |
Multiple Sclerosis |
2 |
330 |
Phase 1, 2 |
2016, 2017 |
Blood |
Celiac Disease |
1 |
204 |
Preclinical |
2016 |
Blood |
Atopic Dermatitis |
2 |
192 |
Phase 2a, 2b |
2018 |
Blood |
Myasthenia Gravis |
1 |
90 |
Phase 2 |
2018 |
DNA |
Peanut Allergy |
1 |
31 |
Preclinical |
2017 |
Blood |
IBD |
1 |
30 |
Preclinical |
2017 |
Tissue |
Diabetes |
2 |
20 |
Research, phase 2 |
2016, 2017 |
Blood, cells |
NASH |
1 |
48 |
Phase 1 |
2018 |
Blood |
Discuss your autoimmune program with our experts who can suggest ideal solutions for your program’s needs
.jpg?width=1920&height=797&name=QB_data_platform_image_desktop%20(1).jpg)
Insight into complex patient biology with biomarker data sciences
Our QuartzBio® enterprise Biomarker Data Management solution centralizes all biomarker data, including exploratory data, preclinical data, and public repositories. Shorten time from data acquisition to data insight and start exploring historically siloed data.