Clinical Sample
Kitting Services
A critical step in the success of any clinical study is the collection and management of clinical samples. Generating quality data from biomarker assays is strongly dependent on the quality of clinical samples collected, and this becomes even more important for studies with complex biomarker programs. Precision focuses on all aspects of clinical kitting to simplify the process of consistent specimen collection.
Custom kit development and biospecimen management to safeguard samples from draw to analysis
Precision’s process to ensure consistent collection and management of clinical samples begins with an understanding of the specific sampling and processing needs required for each type of sample and biomarker analysis to be performed. Customized kitting, collection plans, and logistics plans are developed to meet the needs of the clinical protocol and biomarker analyses.
We then develop clinical site training plans to promote consistent sample collection and manage global shipment, to ensure timely kitting receipt and rapid shipment of clinical samples. The Precision Lab e-Portal provides online, 24/7 access to kit status, shipment status, and sample inventories.
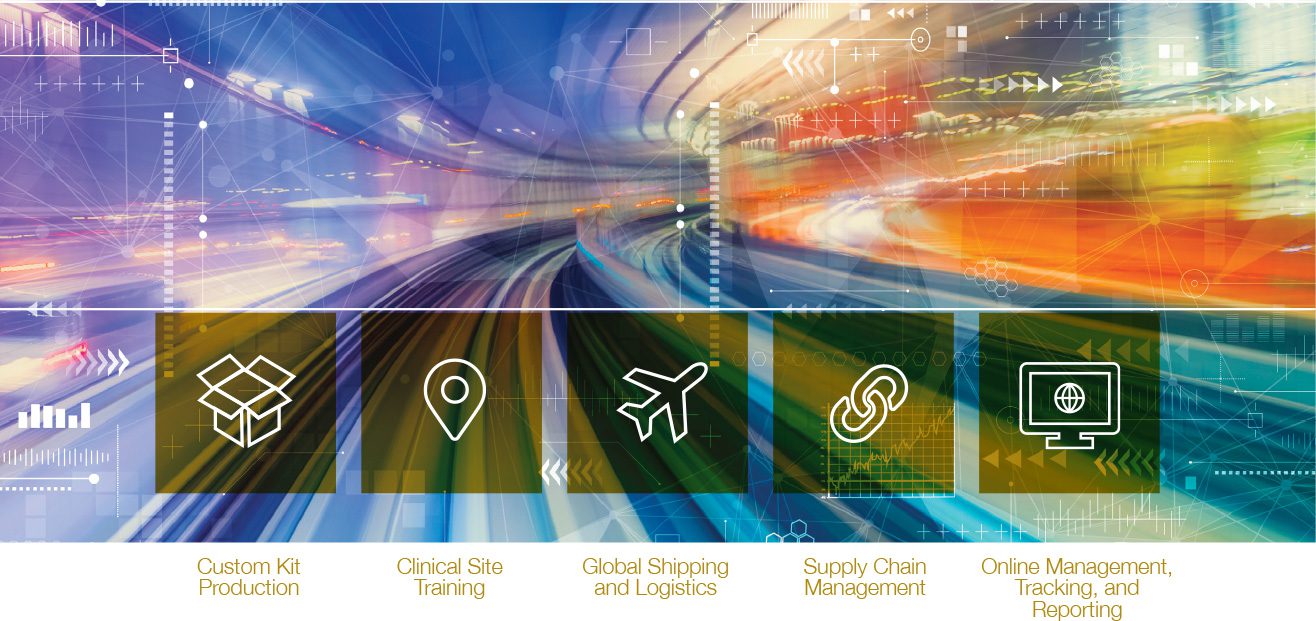

Custom Kit
Production

Clinical Site
Training

Global Shipping
and Logistics
Online Management,
Tracking, and
Reporting
Clinical Kitting Development
Customized kits are built from a purpose-designed kitting facility to ensure rapid construction and shipment, both for study startup and for routine resupply. Kits can be shipped from both the United States and Europe to better accommodate global studies.
Kitting Specifications
- Trial and visit specific
- Multiple sample types
- Traceability of each component and expiry dates via 21CFR Part 11 compliant LIMS
- Detailed kit status reporting
- Sample collection training
- Kit reorder via Lab e-Portal
Clinical Site Training
Effective training of clinical sites is required for consistent sample collection – and is especially important when collection protocols are complex. Precision develops plans, methods, and training plans so that samples are collected the same way across all sites.
- Collection-specific quality assurance plans
- Development of training documentation
- Competency and monitoring programs
Logistics & Transport Management
Planning and designing the optimal route and managing couriers when samples need same-day processing is challenging. Precision’s knowledge in logistics and shipment processes means kits are sent directly to clinical sites, and collected samples quickly arrive at Precision or other facilities for processing, analysis, or storage.
- Export/import permits
- 24-hour staff to receive deliveries and resolve shipment issues
- Knowledge in planning best path for shipment
- Ambient, refrigerated, or frozen shipment options
- Experience with all major couriers – eg, QuickStat, World Courier, FedEx, Marken
Online Tracking via e-Portal
A real-time window into project status is necessary for effective study management. Precision’s Lab e-Portal provides 24/7 access to sample and kit inventories, real-time shipment status, and kit reorder forms so that study needs can be immediately seen and addressed.
Virtual Sample Inventory Management
For more detailed, harmonized information about sample status across sites, labs, and biorepositories, Precision offers virtual Sample Inventory Management via QuartzBio.
Kitting solutions informed by Precision’s deep translational research expertise
Precision’s specialty labs have processed and analyzed millions of clinical samples across every sample type—from DNA and RNA to cells and tissues. Our understanding of the importance of sample quality, and of the inherent needs of biomarker assays, helps us to create a full kitting solution that promotes and aids sample and biomarker analysis for the best possible results in your study.
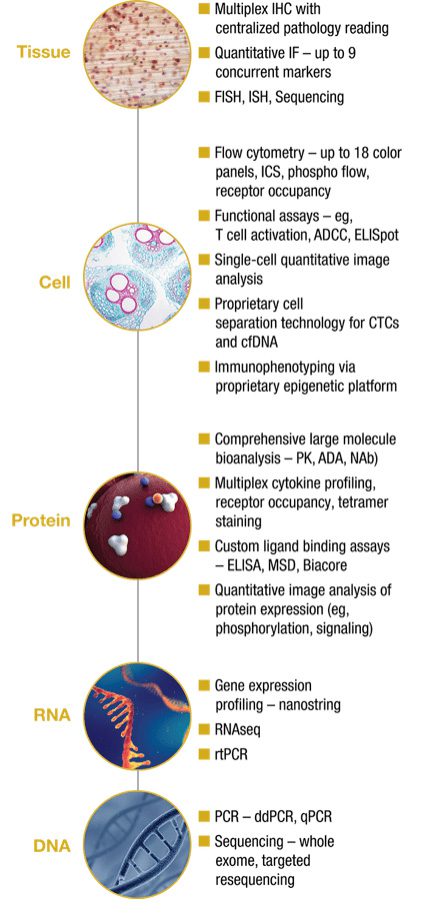
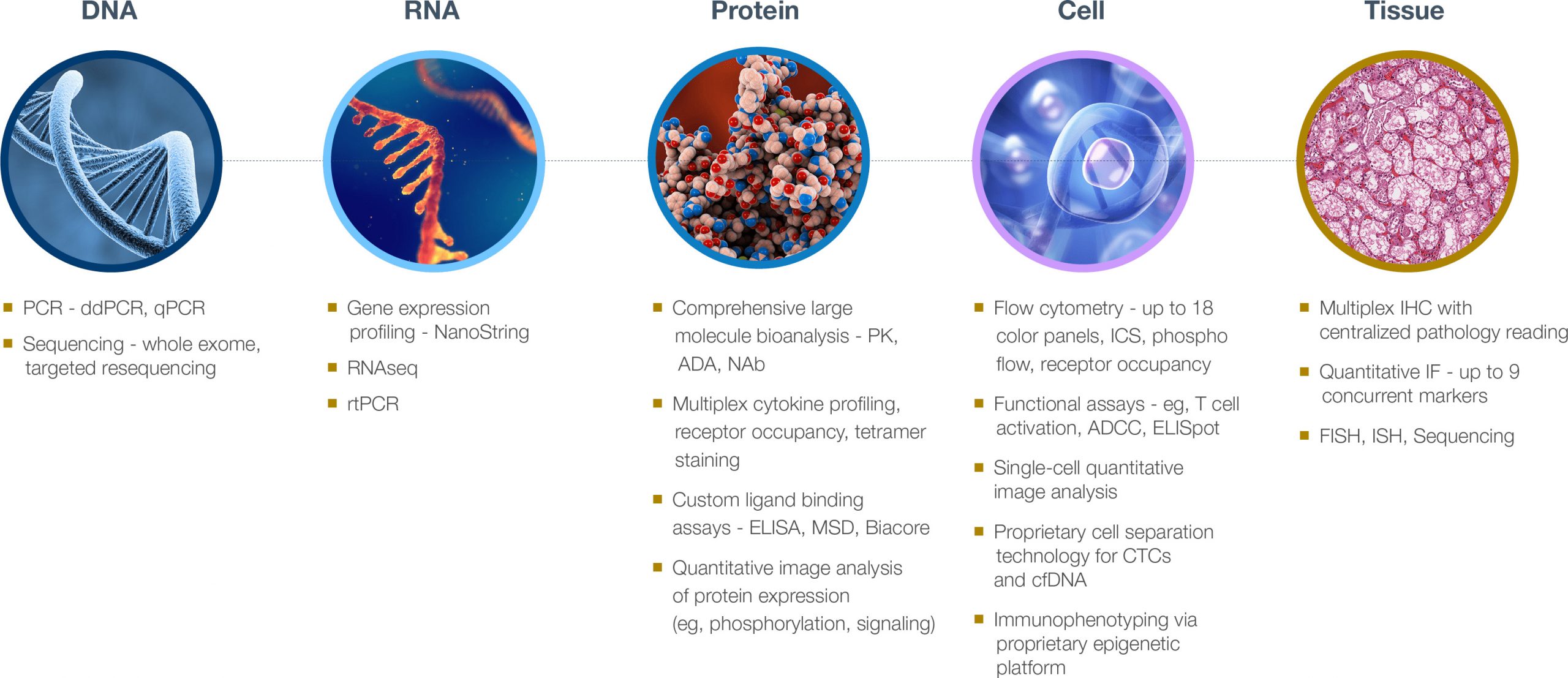
Related services
PBMC Isolations and Clinical Sample Processing
Kit production, clinical site training, and full sample shipment logistics
Biospecimen Management & Biorepository Services
Sample storage under all conditions and temperatures, rapid sample accessioning, and online inventory access
Precision Lab e-Portal
Real-time visibility into project, sample, kit, and shipment status provides 24/7 access to critical study data
