Understanding the Gene Therapy Clinical Trial Landscape
Gene therapy is entering a period of measurable progress. Once viewed as highly experimental, it now underpins active programs in hematology, neurology, ophthalmology, and oncology. Since the first approvals for conditions such as spinal muscular atrophy and inherited retinal disease, sponsors have expanded development into broader, more complex populations. Per Citeline, as of late 2025, approximately 3,200 gene therapy trials are registered globally and in active stages (planned or ongoing). This number includes both industry and academic trials.
Most programs rely on viral vectors, primarily adeno-associated and lentiviral systems, although non-viral approaches are gaining attention as manufacturing and immunogenicity challenges persist. Across therapeutic areas, studies are assessing both in vivo and ex vivo delivery models, with increasing focus on dose durability and long-term monitoring. Oncolytic and gene-modified constructs are also reshaping cancer research, extending the reach of genetic medicine beyond rare disorders.
Regulatory frameworks continue to adapt to these therapies’ unique requirements. Agencies are refining guidance around vector characterization, potency assays, and follow-up duration. Sponsors are also adopting platform-based strategies that enable faster iteration and improved comparability across programs.
This report reviews the current gene therapy trial landscape by phase, geography, and patient segment, highlighting the evolution of study design and delivery approaches in this rapidly maturing field.
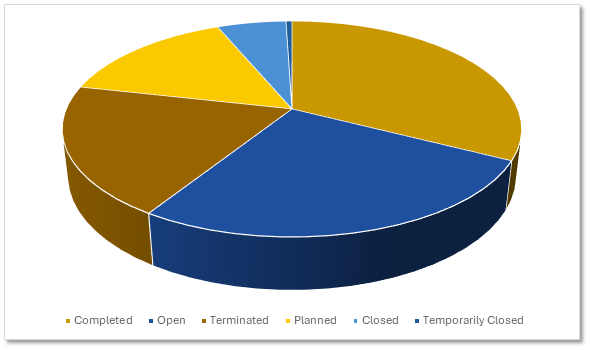
Gene Therapy Clinical Trials by Status
Sponsors are advancing gene therapies aggressively. Roughly the same percentage of open and planned trials have been closed or terminated.
Gene Therapy Trials by Status
Citeline Trialtrove® – 20OCT2025
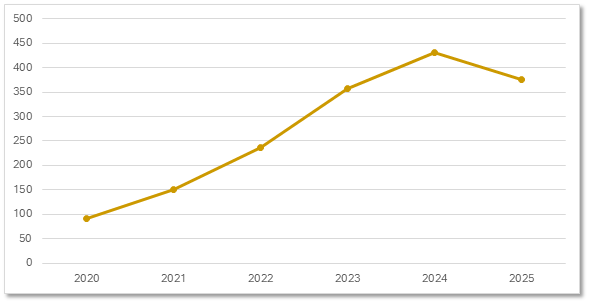
Gene Therapy Trials by Start Date
Start date trends reveal accelerating interest in gene therapy-based modalities.
Planned & Ongoing Gene Therapy Trials by Start Date
Citeline Trialtrove® – 20OCT2025
Gene Therapy Trials by Country
Sponsors are deploying gene therapy trials across diverse markets.
Planned & Ongoing Gene Therapy Trials by Site Country
|
Countries |
Count |
|
China |
940 |
|
United States |
684 |
|
United Kingdom |
143 |
|
Germany |
117 |
|
Spain |
106 |
|
France |
103 |
|
Australia |
101 |
|
Canada |
96 |
|
Italy |
88 |
|
Netherlands |
59 |
Citeline Trialtrove® – 20OCT2025
Gene Therapy Trials by Therapeutic Area
Most of the active gene therapy trials are centered on oncology.
Planned & Ongoing Gene Therapy Trials by Therapeutic Area
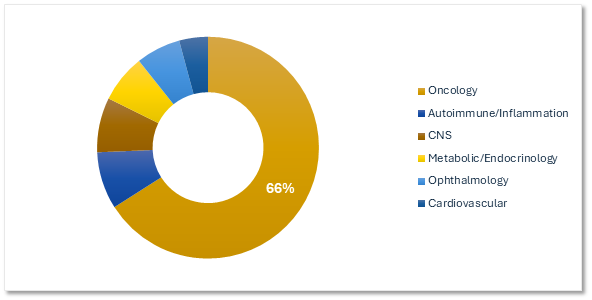
Chart Caption: Citeline Trialtrove® – 20OCT2025
Gene Therapy Trials by Indication
Hematological malignancies are a frequent target in current cell therapy trials, taking 4 of the top 6 indication spots.
Planned & Ongoing Gene Therapy Trials by Indication
|
Indication |
Count |
|
Lymphoma, Non-Hodgkin's |
363 |
|
Unspecified Solid Tumor |
221 |
|
Leukemia, Acute Lymphocytic |
202 |
|
Multiple Myeloma |
187 |
|
Lung, Non-Small Cell |
123 |
|
Leukemia, Acute Myelogenous |
115 |
|
Ovarian |
107 |
|
Lupus |
104 |
|
Pancreas |
102 |
|
Colorectal |
95 |
Citeline Trialtrove® – 20OCT2025
Gene Therapy Trials by Phase
Most current gene therapy research is in the early phase.
Planned & Ongoing Gene Therapy Trials by Phase
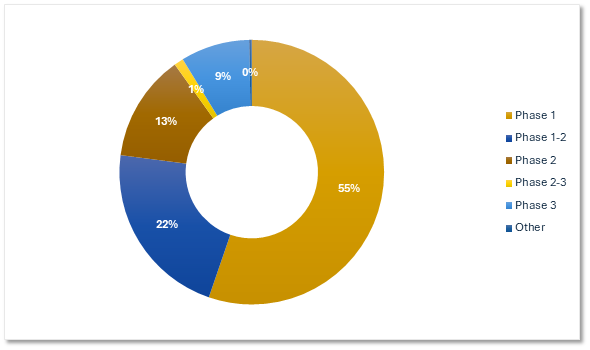
Citeline Trialtrove® – 20OCT2025
-

Gene Therapies - Early Phase Research - Case Study
Case Study: Rapid Startup Phase 1 Cardiomyopathy CRISPR Gene Therapy
- |
Precision's Expertise in Gene Therapy Research
Precision has varied experience in gene therapy.
Gene Therapy Trial Starts
For the last 5 years, the number of our gene therapy trials has trended upwards.
Precision for Medicine's Gene Therapy Trials by Start Date
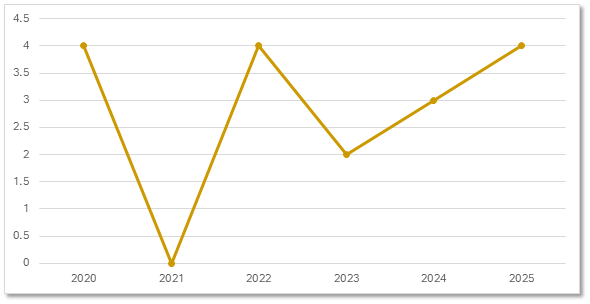
Citeline Trialtrove® – 20OCT2025
Precision's Reach in Gene Therapy Research
We have conducted gene therapy trials in diverse locations, figuring prominently in the United States, Australia, and Canada.
Precision for Medicine's Gene Therapy Trials by Site Country
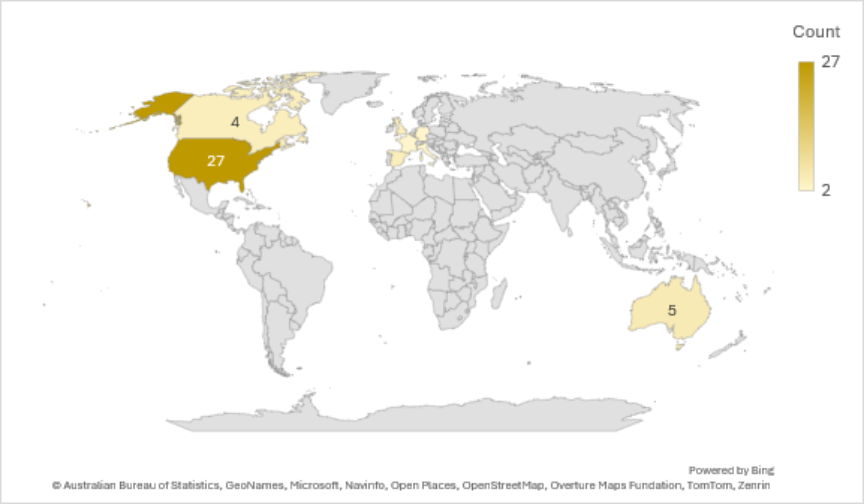
Citeline Trialtrove® – 20OCT2025
Gene Therapy Trials by Phase
Our portfolio includes a strong emphasis on early development.
Precision for Medicine's Gene Therapy Trials by Phase
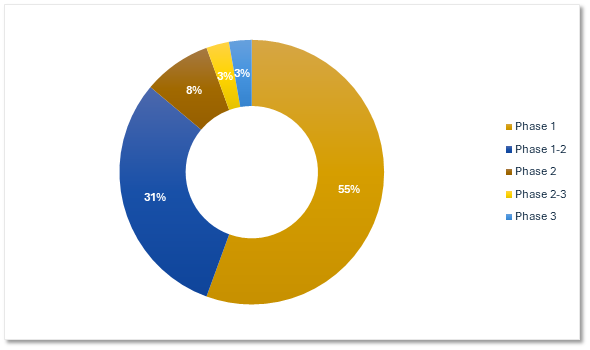
Citeline Trialtrove® – 20OCT2025
Precision's Experience by Therapeutic Area
Most of our gene therapy experience is in oncology.
Precision for Medicine's Gene Therapy Trials by Therapeutic Area
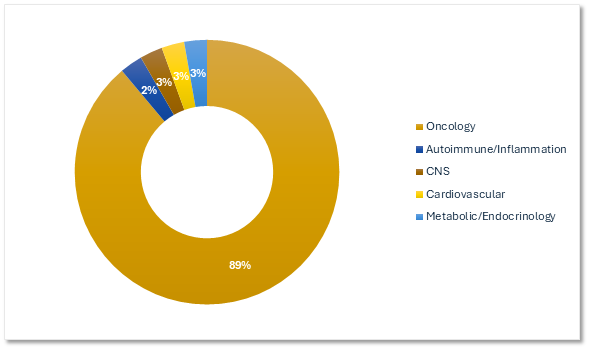
Citeline Trialtrove® – 20OCT2025
Precision Gene Therapy Trials by Top Indications
Precision has conducted a variety of hematological malignancies and solid tumor gene therapy trials.
Precision for Medicine's Cell Therapy Trials by Top Indications
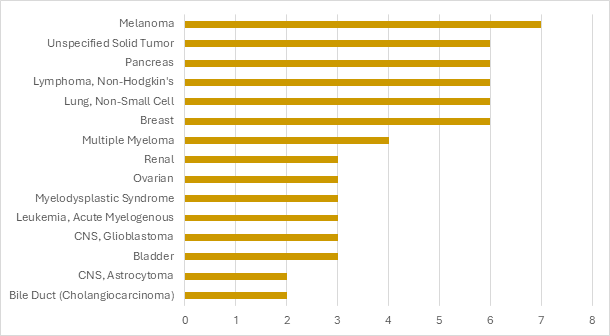
Citeline Trialtrove® – 20OCT2025
Understanding the Difference Precision Makes
Gene therapy development requires close coordination between preclinical, manufacturing, and clinical teams. Precision’s interdisciplinary teams have been built to advance research and development, clinical, manufacturing and commercialization solutions to help our innovative sponsors transform patient lives. We know that delivering an advanced therapy to market requires a comprehensive and integrated approach.
Precision for Medicine supports sponsors through this continuum, integrating operational and scientific oversight, our oncology and rare disease experience enables sponsors to manage the specific demands of gene therapy programs while maintaining quality, safety, and regulatory alignment.