Immunohistochemistry (IHC) Services
Immunohistochemistry (IHC) biomarkers are essential to drug development. At Precision, we maximize data from limited tissue using our high-quality repository to develop, validate, and implement IHC assays for exploratory through late-phase studies. For trials needing spatial context beyond chromogenic IHC, our mIF workflows enable single-cell phenotyping and proximity analysis.
Precision can support utilization of IHC across preclinical through clinical development, with support for IHC based Companion Diagnostics (CDx)
- Global Laboratories
- Platform Agnostic
- Full Life Cycle Support
-
Precision brings you access to 3 labs with immunohistochemistry capabilities
With 2 labs located in the United States and 1 lab located in Europe – Precision's reach extends globally for IHC solutions.
- Houston, Texas, United States
- Winston-Salem, NC, United States
- Royston, UK
.jpg?width=1366&height=768&name=iStock-1401326975%20(1).jpg)
-
Access to all major IHC platforms
Leica, Dako, and Ventana staining platforms are available at our 3 global IHC labs, with redundancy of instrumentation within and across labs, supporting development of assays or diagnostics based on any platform.

-
From preclinical through late phase studies
Explore IVD & CDx regulatory consultingPrecision's team of IVD & CDx Regulatory Consulting experts provide full lifecycle development support to construct plans for any eventuality as your project progresses.
Our-site pathologists are available for custom tissue annotation and analysis, as well as to support custom tissue sourcing.

Target the clinical biomarkers important in your trials
See which biomarkers we've worked with and some we've for immunochemistry and multiple immunofluorescence.
/frozen-tissue.webp?width=2000&height=1333&name=frozen-tissue.webp)
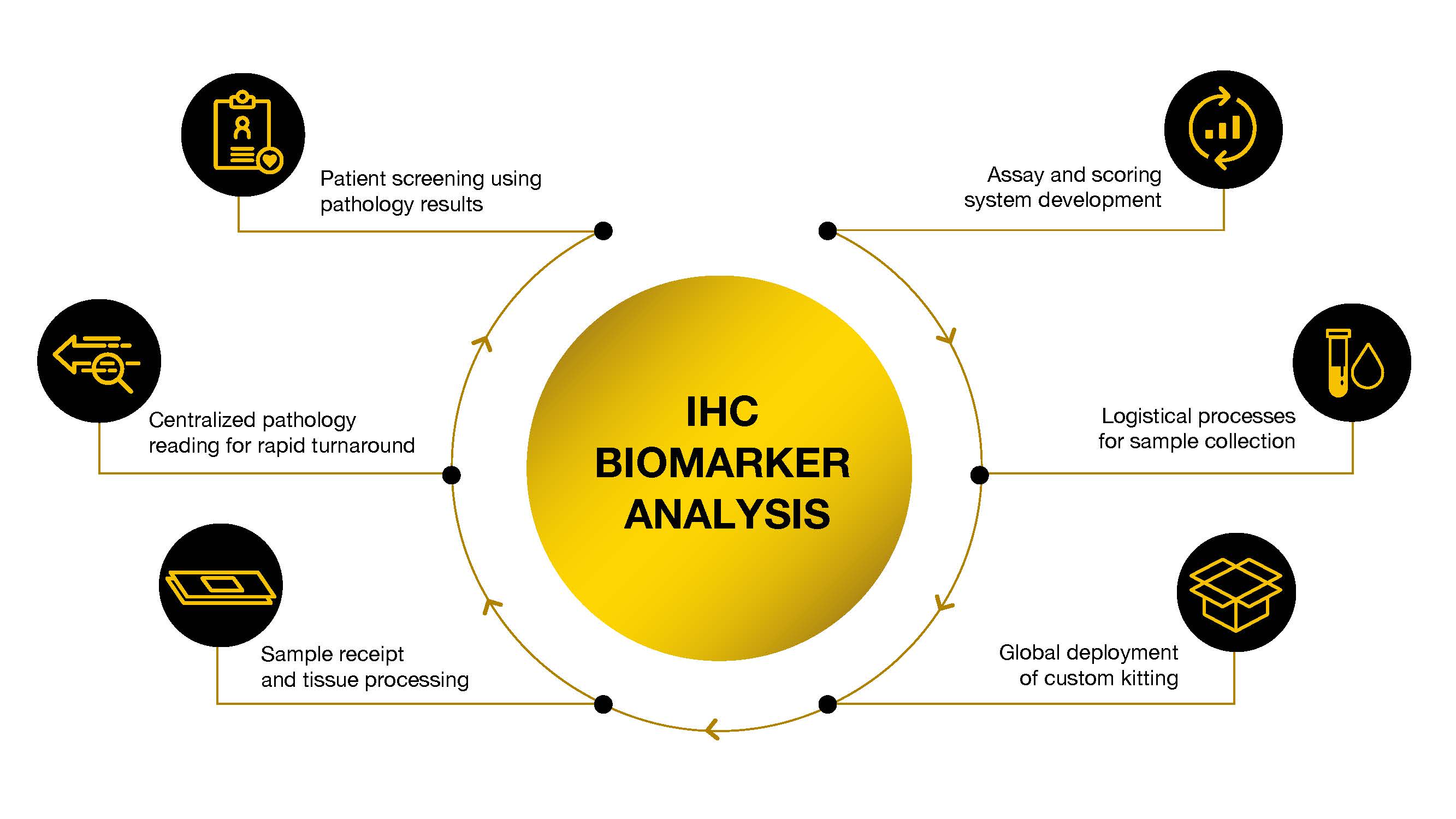
Experts in all aspects of IHC biomarker analysis including:
- Custom assay development, qualification, transfer, & validation, including scoring system, using our tissue archive
- Logistical process for sample collection
- Global deployment of custom kitting
- Centralized pathology reading with manual scoring or AI-assisted scoring using HALO AP and Proscia Concentriq.
- Real time analysis for patient screening & enrollment (CLIA)
- Development of an IHC assay into a companion diagnostic (CDx)
- Sample receipt and wet tissue processing
Case Study
Patient stratification using biomarkers from biopsies
Precision developed an IHC assay for patient study enrollment by P-Cadherin expression in tumor biopsies:
- Assay development and customized kitting generation and shipment
- Development of a semi-quantitative IHC scoring index (SI) to compare P-Cadherin staining intensity and prevalence from sample to sample
- IHC pathology analysis by scoring index; rapid turnaround of results reported within 72 hours of receipt
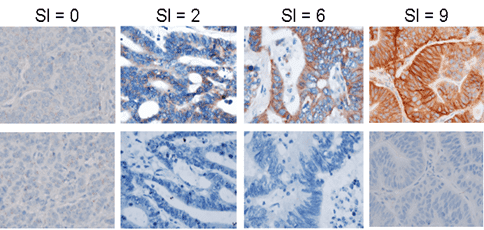
This rapid turnaround of scoring index data enabled P-Cadherin expression to be utilized as a criterion for inclusion in the study.
Integrating biomarker and clinical trial data to help researchers understand and interpret biomarker assay data in the context of other assays as well as the clinical trial data, Precision provides the option of managing and analyzing data using our proprietary QuartzBio enterprise Biomarker Data Management software solution.
Discover how Precision’s suite of services can accelerate your biomarker development
Precision has experience in developing and validating IHC biomarkers to support many key areas of research in our CAP accredited CLIA laboratory, with the capabilities to:
Evaluate pharmacodynamic effects and therapeutic efficacy on protein expression
Accelerate patient enrollment and stratification with a 72-hour turnaround time
Understand drug activity via mechanism of action
Identify predictive and prognostic biomarkers
Monitor immune response to immunotherapies by profiling key immune cell populations
Multi-service integrations
Clinical biomarkers go far beyond traditional IHC – couple IHC biomarker assays with mIF for spatial context and single-cell insights, or integrate with genomics, liquid biopsy, and flow cytometry for richer datasets.
-
Explore


Multiplex Immuno-fluorescence
ExploreAutomated multiplex immunofluorescence services that enable quantitative visualization of up to 9 markers in tissue and liquid biopsies
Multiplex Immuno-fluorescence
ExploreAutomated multiplex immunofluorescence services that enable quantitative visualization of up to 9 markers in tissue and liquid biopsies -
Explore
/iStock-1257429592%20(2)%20(1).jpg?width=396&height=416&name=iStock-1257429592%20(2)%20(1).jpg)

Liquid Biopsy
ExploreAn alternative when tissues are not available for serial assessment of pharmacodynamic biomarkers

Liquid Biopsy
ExploreAn alternative when tissues are not available for serial assessment of pharmacodynamic biomarkers
-
Explore


Genomics Services
ExploreWith NGS, NanoString, qPCR and ddPCR capabilities, we can add insights from genomic and transcriptomic technologies to your understanding of patient biology.
Genomics Services
ExploreWith NGS, NanoString, qPCR and ddPCR capabilities, we can add insights from genomic and transcriptomic technologies to your understanding of patient biology. -
Explore


Flow Cytometry
ExploreStandard and spectral flow cytometry, on both research-grade and CLIA-validated instruments
Flow Cytometry
ExploreStandard and spectral flow cytometry, on both research-grade and CLIA-validated instruments -
Explore


FISH/ISH
ExploreDevelopment of multiplex FISH/ISH assays to detect abnormalities in a range of tissues, including hematological and solid organ tumors
FISH/ISH
ExploreDevelopment of multiplex FISH/ISH assays to detect abnormalities in a range of tissues, including hematological and solid organ tumors

Maximize data from your tissue samples with IHC
Find the samples needed for your IHC analysis with Precision’s tissue biospecimen repository
Our Related Biospecimens
-
Explore
%20Tissue/FFPE-Tissues-hero.png?width=396&height=416&name=FFPE-Tissues-hero.png)

Formalin-Fixed Paraffin Embedded (FFPE) Tissue
ExploreExtensive inventory of pathologist-reviewed formalin-fixed paraffin-embedded tissue blocks & slides from normal and diseased subjects.

Formalin-Fixed Paraffin Embedded (FFPE) Tissue
ExploreExtensive inventory of pathologist-reviewed formalin-fixed paraffin-embedded tissue blocks & slides from normal and diseased subjects.
-
Explore


Liquid Biopsy
ExploreAn alternative when tissues are not available for serial assessment of pharmacodynamic biomarkers

Liquid Biopsy
ExploreAn alternative when tissues are not available for serial assessment of pharmacodynamic biomarkers