Small Cell Lung Cancer (SCLC) accounts for roughly 10–11% of all lung cancer cases [1] globally, yet it remains one of the most aggressive and difficult-to-treat subtypes
With rapid progression and limited second-line options, SCLC trials face intense pressure to identify, screen, and enroll patients before disease advancement closes the therapeutic window. In this case study, we explore how a sponsor’s Phase 2–3 trial overcame feasibility challenges through targeted site selection and real-time recruitment analytics—ultimately achieving full enrollment across 26 countries.
|
Therapeutic Area |
Oncology |
|
Indication |
Small Cell Lung Cancer (SCLC) |
|
Study Phase |
2-3 |
|
Patient Segment |
Adult, Second Line |
|
Patients |
480+ |
|
Sites |
195+ |
|
Countries |
26: Australia; Bulgaria; Canada; France; Georgia; Hong Kong, Hungary; India; Italy; Lithuania; Malaysia; Philippines; Poland; Russia; Serbia; Slovakia; South Korea; Spain; Taiwan, Thailand; Ukraine; United Kingdom; United States |
|
Design |
Open-label, randomized study comparing IP + comparator vs. comparator alone in relapsed/refractory SCLC |
The SCLC Recruitment Challenge
This multinational trial faced three major hurdles:
- Compressed timelines: Patients needed to be screened and dosed within days of relapse
- Global variability: Referral pathways and incidence rates varied widely across regions
- High screen failure rates: Comorbidities and prior therapies often disqualified otherwise eligible patients
To address these, the sponsor partnered with Precision for Medicine to deploy a dynamic feasibility model and a flexible site activation strategy.
Precision Feasibility in Action
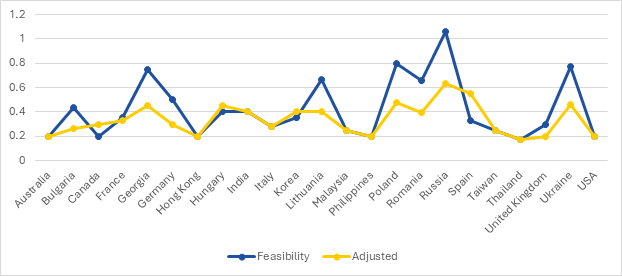
Initial projections were built using historical enrollment data, regional incidence, and site-level performance. As the trial progressed, real-time adjustments were made based on actual screening and randomization trends.
A dual-line graph tracked projected feasibility versus adjusted actuals, enabling:
- Resource reallocation to high-performing regions
- Deprioritization of underperforming sites
- Sustained enrollment velocity despite early screen failures
Spain Leads Global Recruitment
Of the 480+ patients enrolled globally, Spain alone contributed 73 — over 15% of total enrolled. This success was driven by:
- Early site activation and streamlined contracting
- Strong investigator engagement
- High patient identification rates
Other notable contributors included the United States (64 enrolled) and Russia, Korea, France and Georgia, each with smaller but consistent enrollment.
Top-Performing Sites
The trial’s success hinged on a handful of standout sites. In Spain and the United States, investigators led the charge. The most successful sites shared key traits:
- Oncology specialization with SCLC experience
- Efficient pre-screening workflows
- Strong patient trust and referral networks
Lessons Learned
- Feasibility is dynamic: Static models can’t keep pace with real-world variability. Real-time data monitoring enabled agile decision-making.
- Site selection matters: A few high-performing sites can drive the majority of enrollment—if activated early and supported well.
- Global doesn’t mean slow: With the right strategy, multi-country trials can outperform single-region studies in both speed and diversity.
In SCLC, Precision can mean the difference between delay and delivery
This trial proved that even in rare, aggressive indications, global studies can succeed when feasibility is treated as a living model—not a static spreadsheet. With the right sites, the right data, and the right support, precision becomes more than a strategy—it becomes a standard.
References
- Terrasse V. Global lung cancer incidence according to subtype: new study highlights rising adenocarcinoma rates linked to air pollution. International Agency for Research on Cancer. February 3, 2025. https://www.iarc.who.int/wp-content/uploads/2025/02/pr361_E.pdf. Accessed December 1, 2025.


.png?width=396&height=224&name=Streamlining%20Radiopharmaceutical%20Clinical%20Trials%20Precision%20Case%20Study%20(2).png)

