
Flow Cytometry in Cell Therapy
Flow cytometry’s high-throughput analysis of large cell populations with small sample-batch requirements makes it highly attractive for cell and gene therapeutic development and validation, fitting comfortably within the field of PK/PD and biomarker assays.
Complexities of cell therapy programs present not only scientific but also unique technical hurdles. Precision for Medicine has the expertise to help navigate these challenges with the development of your cell therapy product and clinical program.
Measure a range of cell therapy development endpoints with flow cytometry
Preparing and Characterizing Donor Samples
- Evaluating CAR expression on therapeutic cells.
Monitoring Therapeutic Effects in Patients
- CAR T-cell/CAR NK-cell persistence and therapeutic effect
- Enumeration and characterization of cell therapy product
- Antidrug antibodies (ADA) and immunogenicity
- Depletion of a target population by a cell therapy product
Assessing Cell Functionality and Potency of Immune System Response or Cell Therapy/CAR
- Estimation of effector cell activation
- Measurement of markers of cell exhaustion
- Expanded phenotypic analysis of marker expression
- Intracellular cytokine expression
Phenotype CAR T cells with flow cytometry
Precision routinely runs complex assays for cell therapy trials, including phenotypical characterization of CAR T cells in clinical samples. Supporting assay development and validation are readily available.
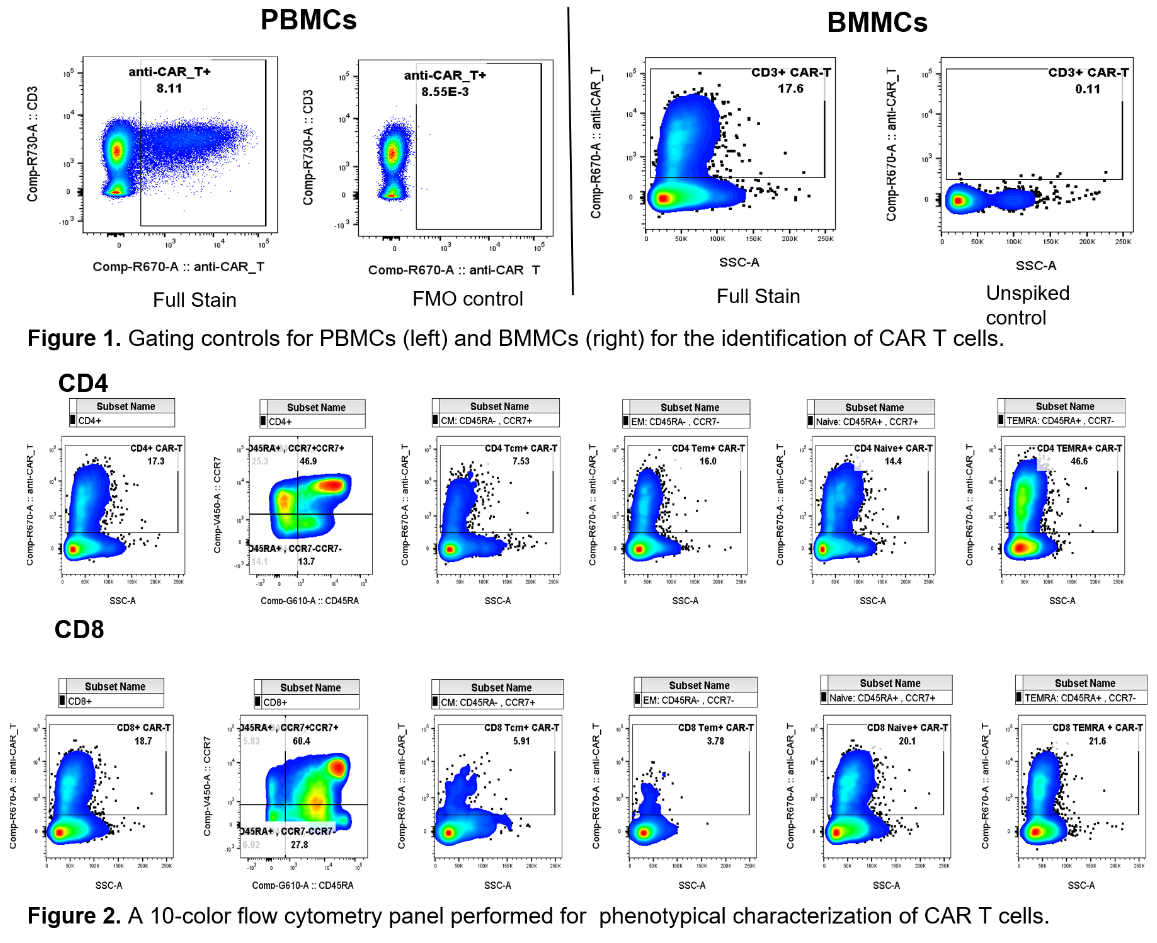
- CAR T cells are spiked into healthy donor peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs).
- Develop gating strategy: fluorescence minus one (FMO) control staining and unspiked samples were used as gating controls for PBMCs and BMMCs, respectively (Fig. 1).
- Detect and characterize the CAR T cells with a 10-color flow cytometry panel (Fig. 2).
Your entire cell therapy development journey, start to finish
Partner with Precision for your adoptive cell therapy testing needs—wherever you are in development
- Clinical trial management
- Scientific expertise
- Biospecimens
- Manufacturing
-
Leverage our trial management expertise
Explore cell therapy trial management capabilitiesPrecision runs cell therapy trials in 26+ countries and 5 regions: 11 active cell therapy trials in both autologous and allogenic cell therapies. We work on each aspect of a cell therapy trial, including
-
Protocol review and development
-
Biomarker strategy development and implementation
-
SAP review
-
Clinical/project management
-
Logistical coordination through product life cycle
-
Medical monitoring

-
-
Custom assays to measure your cell therapy response
Consult with us to choose the best-fit platforms and approaches for measuring the endpoints critical to your advanced therapy development. Monitor PD response, assess safety, and identify targets for patient selection/predictive biomarkers.
- PK assays by ddPCR/qPCR and flow cytometry
- Cell therapy persistence by flow cytometry
- ADA by flow cytometry and ligand binding assays (LBA/ELISA)
- Immunophenotyping by flow cytometry (T-cell lineage, B cells, memory T cells, Treg)
- Multiplex immunofluorescence for tumor infiltration by CAR T/CAR NK cells, cell therapy product
- Immune cell monitoring in blood and tissue with qPCR (Epiontis ID®)
- ELISpot for antigen-specific testing in cell therapies
.jpg?width=2000&name=iStock-1090255620%20(2).jpg)
-
High-quality clinical samples
Learn more about BiospecimensEnsure assay-to-assay consistency by leveraging our carefully curated product portfolio for your cell therapy studies. Precision provides access to specimens containing high yields of single-donor cells derived from our leukopak products, collected under IRB-approved protocols and QC’d post-collection.
- Leukopaks are collected via apheresis and contain high yields of cell populations found in the peripheral blood—such as T cells, monocytes, or NK cells
- Research grade or GMP grade available
- Mobilized leukopaks are collected via apheresis from donors treated with FDA-approved G-CSF drugs, resulting in high yields of CD34+ stem cells
- Isolated primary cells
- Specific cell populations isolated from leukopak products and shipped directly to your lab for downstream applications

- Leukopaks are collected via apheresis and contain high yields of cell populations found in the peripheral blood—such as T cells, monocytes, or NK cells
-
Strategy and operational execution for advanced therapies
Explore manufacturing solutions for cell therapy trialsBuild GMP-compliant gene and cell therapy facilities from 5000 ft2 to over 500,000 ft2, using cutting-edge modular, emerging bioreactor and single-use technologies. Our expertise includes
- Turnkey capital projects
- Tech transfers and facility builds
- Validation and regulatory compliance
- Quality management systems (early phase through commercial) with quality control/release testing


Accompanied by comprehensive regulatory consulting
Precision for Medicine is a regulatory leader in industry with expertise in emerging fields, including gene therapy CDx, with extensive knowledge in NAb and TAb assay development, supporting 14 different rare disease pipelines. Globally situated, Precision can conduct testing in laboratories in both US and Germany.
With 125+ years of cumulative regulatory experience, our team provides end-to-end regulatory solutions and support with industry knowledge developing global regulatory strategies, CLIA- and CLSI-compliant analytical validation study designs, and clinical trials enabling regulatory submissions, in addition to marketing authorization regulatory filings globally.
Frequently asked questions
Why are cell-based therapies, such as CAR T and NK cells, challenging to develop?
Cell-based therapies, such as CAR T and NK cells, are highly customized and vary greatly based on product attributes, each demanding rigorous regulatory approval. It is therefore crucial to find a partner who offers access to a robust scientific and regulatory network and integrates the various elements of your program to achieve your milestones.
Explore our cell therapy expertise
Explore related resources
Explore all resourcesValues of Precision flow cytometry
-
Established experience
Established experience running complex flow cytometry in gene therapy, cell therapy, immuno-oncology therapies, validating up to CLIA -
Senior staff
Sample testing by experienced staff who understand the disease biology and gating strategy -
Quality control
Real-time Quality Control during the run to ensure sample integrity
Enhance your clinical results with complementary lab services
-
Explore


Epiontis ID® Epigenetic Cell Profiling
ExplorePrecision’s proprietary immune monitoring technology which delivers robust, repeatable, and cost-effective immune cell phenotyping on any sample type
Epiontis ID® Epigenetic Cell Profiling
ExplorePrecision’s proprietary immune monitoring technology which delivers robust, repeatable, and cost-effective immune cell phenotyping on any sample type -
Explore


Cytokine Profiling & Proteomics
ExploreCustomized assays to detect cytokines or proteins of interest utilizing ELISA, Luminex, MSD, and Western Blotting

Cytokine Profiling & Proteomics
ExploreCustomized assays to detect cytokines or proteins of interest utilizing ELISA, Luminex, MSD, and Western Blotting
-
Explore


ELISpot and Fluorospot
ExploreELISpot and FluoroSpot - including custom assays in support of gene therapy immunogenicity - from preclinical through clinical development

ELISpot and Fluorospot
ExploreELISpot and FluoroSpot - including custom assays in support of gene therapy immunogenicity - from preclinical through clinical development