

Neutralizing Antibody (NAb) & Total Antibody (TAb) Assays
Precision has extensive experience in developing neutralizing antibody and total antibody assays to measure pre-existing antibodies to gene therapy viral vector-based therapeutics across multiple types of vectors and capsid types, and can support development of gene therapy NAb/TAb assays into commercial CDx
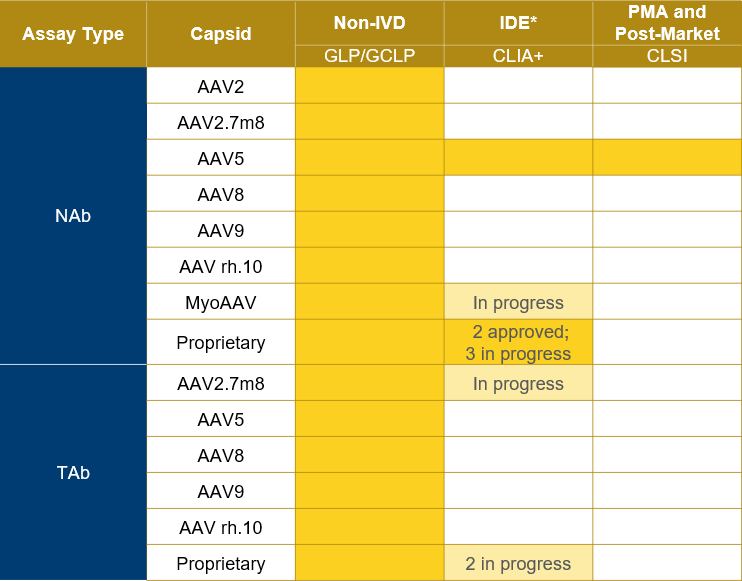
Expertise in NAb/TAb assays for gene therapy
Precision for Medicine's scientific team has developed and validated both NAb and TAb assays in both humans and NHP for several capsid types.
In addition, Precision has run >8,000 samples on GxP-validated assays and continues to support the most prominent companies developing gene therapies.
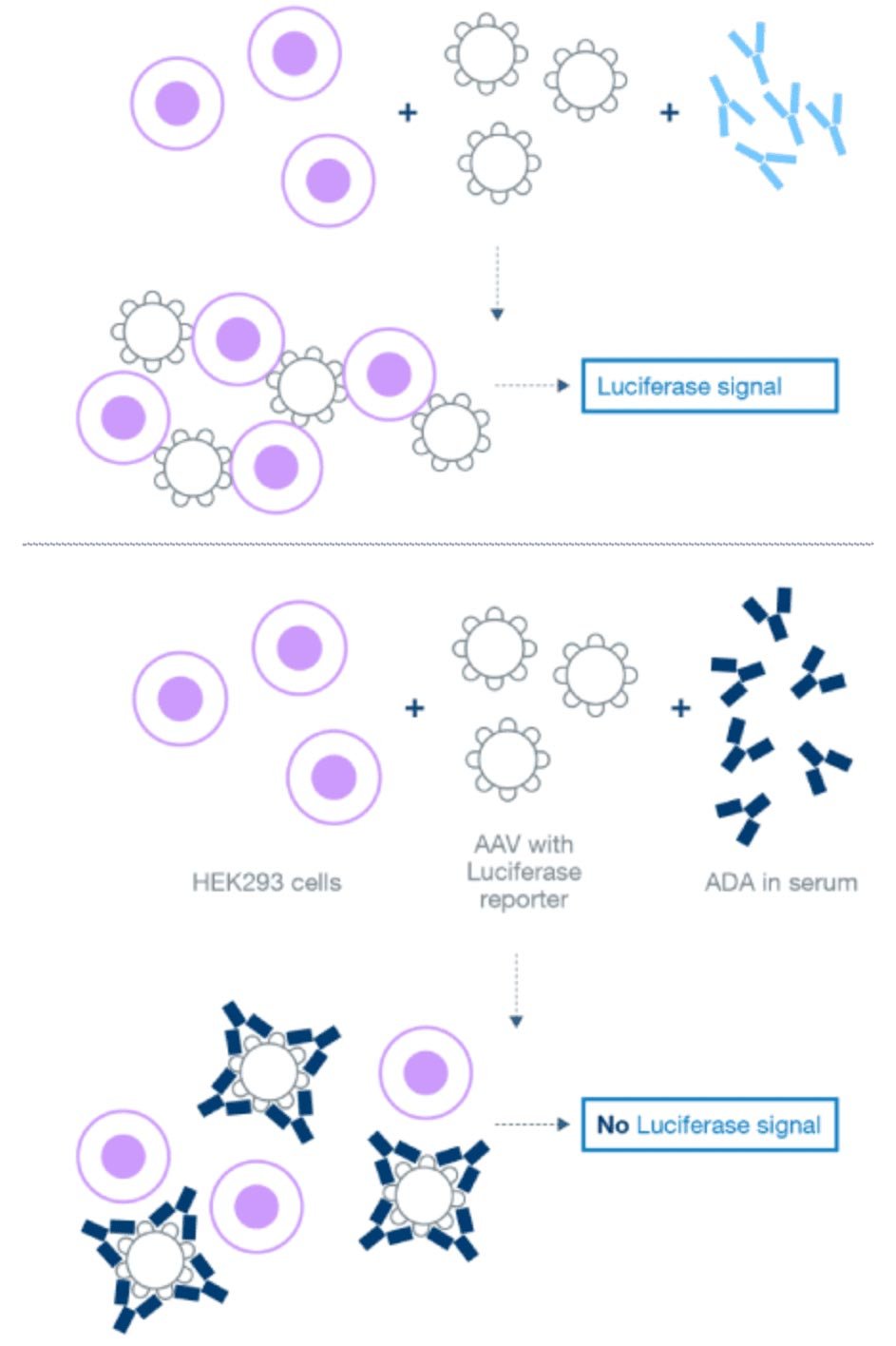
Development of a NAb CDx for a gene therapy
Problem
Because preexisting antibodies may hamper transduction efficiency and reduce efficacy of AAV5 vector-based therapy, the sponsor wanted us to develop and validate an AAV5 NAb CDx to identify patients most likely to benefit from treatment.
Solution
We developed and optimized a cell-based luciferase reporter assay that could detect and measure anti-AAV5 capsid antibodies capable of neutralizing viral transduction of HEK 293 cells. This project included maintenance of the cell bank, running the assay, and testing clinical samples.
We are also developing a single-site CDx using our ISO 13485-certified and FDA 21 CFR 820-compliant laboratory facilities. As of early 2019, this project included full regulatory, biostatistical, and commercial services for the CDx, including both strategic and tactical support for all activities throughout the product life cycle.
Expert insights: assay development to CDx approval
Read peer-reviewed articles authored by our Precision team on best practices in this field.
-
Research Article
AAPS Recommendations for the Development of Cell Based Anti Viral Vector Neutralizing Antibody Assays (2020)
Download Article
-
Research Article
AAPS Evaluation of Cellular Immune Response to Adeno-Associated Virus Based Gene Therapy (2023)
Download Article
-
Research Article
AAPS Evaluation of the Humoral Response to Adeno-Associated Virus Based Gene Therapy Modalities Using Total Antibody Assays (2021)
Download Article
-
Research Article
The Regulation of Companion Diagnostics A Global Perspective. Ther Innov Regul Sci. 2013
Download Article
Regulatory solutions for NAb/TAb CDx
Precision also support development and commercialization of total antibody and neutralizing antibody companion diagnostics, including:
- FDA diagnostic pre-submission and modular PMA submissions
- Complete LDT analytical studies under full design control
Our experience speaks for itself-we’ve already successfully completed over 100 CDx regulatory filings globally
Related Services
-
Explore


Large Molecule PK
ExploreDevelopment, validation, and implementation using ELISAs and MSD assays for biologics, flow cytometry and ddPCR for cell-based therapies
Large Molecule PK
ExploreDevelopment, validation, and implementation using ELISAs and MSD assays for biologics, flow cytometry and ddPCR for cell-based therapies -
Explore


MesoScale Discovery (MSD)
ExploreMeasuring a range of analytes in complex sample matrices consistently and at high sensitivity, with applications including ADA, NAb, TAb, and PK assays
MesoScale Discovery (MSD)
ExploreMeasuring a range of analytes in complex sample matrices consistently and at high sensitivity, with applications including ADA, NAb, TAb, and PK assays -
Explore


ELISA
ExploreServices using the gold standard for quantitative antibody-based bioanalysis, with specialization in immunogenicity assay development
ELISA
ExploreServices using the gold standard for quantitative antibody-based bioanalysis, with specialization in immunogenicity assay development
