Solving the Unique Central Lab Services Challenges of Precision Medicine Trials
Drug development requires significant investment, not just in dollars, but also in effort and expertise. In today’s precision medicine landscape, where biomarker-driven clinical trials and studies with complex designs are becoming more common, it is increasingly difficult to coordinate effectively to ensure a successful study.
Conduct of these clinical trials requires proactive planning and broad expertise not just in study execution and laboratory analysis, but also in sample collection, logistics, and management. This is a significant undertaking due to the complexity in sample collection, processing, logistics, storage, and sample data management involved in complex and biomarker-driven clinical trials. This presents a need for central lab services solutions tailored for precision medicine trials.
Complex Ecosystem of Biomarker-Driven Trials
In the current paradigm of drug development, clinical trials often involve a proliferation of sites, some of which are quite small but require the same level of management as larger institutions. These sites feed into a central lab, or sometimes multiple central labs, which then distribute samples to screening and specialty labs which perform the assays needed to generate data on the defined and exploratory biomarkers of interest.
Each of these entities—every site and every central, screening, or specialty lab—represents a node in a complex ecosystem. Each node requires contracting, sample collection kits, chain of custody traceability, and data generation. All of this adds up to a substantial task of management, coordination, and data consolidation, beyond what is already required for biomarker identification, study design, trial execution, regulatory filings, and other development activities.
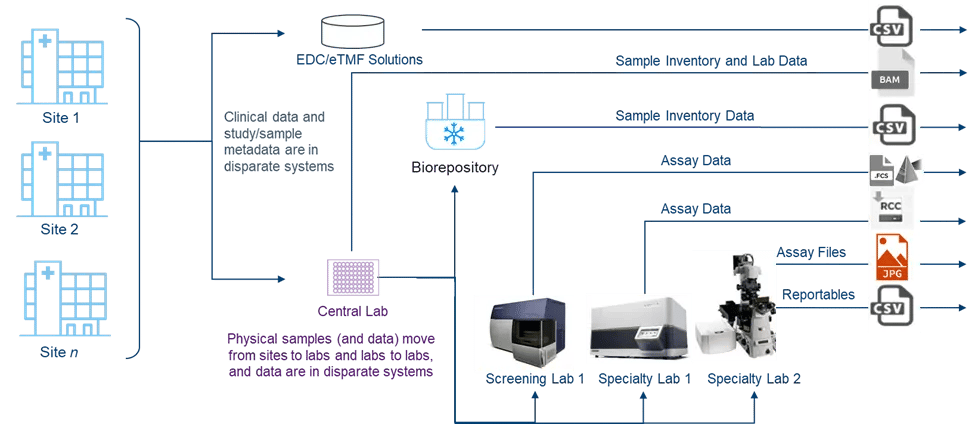 Figure 1. The complex, disconnected ecosystem of biomarker-driven trials leads to sample and data chaos
Figure 1. The complex, disconnected ecosystem of biomarker-driven trials leads to sample and data chaos
Compounding the complexity, the clinical trial ecosystem is often disconnected, creating operational and scientific challenges. While clinical data is stored in an Electronic Data Capture (EDC) or Electronic Trial Master File (eTMF), samples and their associated data move from sites to multiple labs in disparate systems and different formats. This increases the importance of accurate sample management for ensuring adherence to both the study protocol and the informed consent form (ICF).
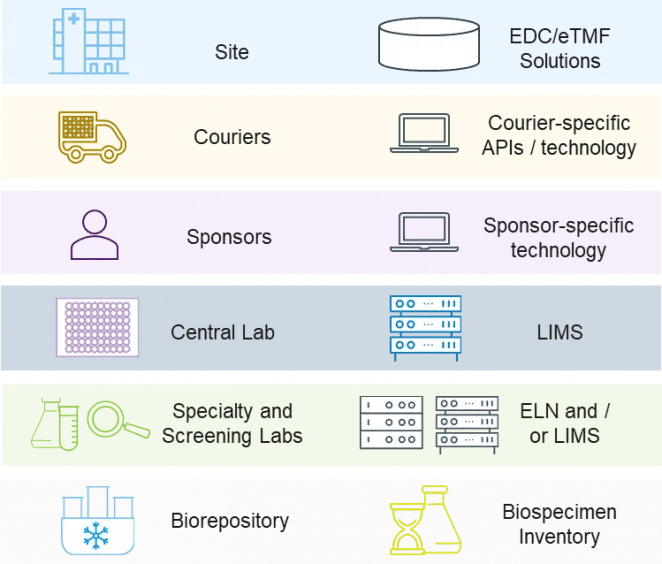 Figure 2. Sample management involves multiple stakeholders and myriad data systems
Figure 2. Sample management involves multiple stakeholders and myriad data systemsStakeholders in Sample Management
Sample management involves numerous stakeholders, each with their own system of generating and capturing data:
Sites need to have sample collection supplies on hand and are responsible for ensuring that samples are collected in accordance with the study protocol. Sample collection data is captured in an EDC or eTMF solution.
Couriers are responsible for transporting samples from sites to labs in a manner that is both visible and suitable for the sample requirements with respect to time, temperature, condition, and import/export regulations. Most couriers have their own application programming interface (API) or technology for data capture.
Central labs take on the primary responsibility of ensuring that chain of custody is maintained and appropriately documented from the sample collection site to each downstream control point. Data on sample type, de-identified participant information, sample conditions, labels, site, collection time, and more is collected in a laboratory information management system (LIMS). Depending on the study, central labs may also be responsible for:
- Conducting laboratory assessments
- Compiling test reports
- Contracting couriers for delivering and receiving kits and samples
- Producing kits, training sites on how to use them correctly, and managing kit expiry / inventory
- Processing samples
- Performing sample accessioning and logistics management
- Sending samples to specialty and screening labs for testing
Screening and specialty labs perform assays as per the study protocol and collect data in an electronic lab notebook (ELN) or LIMS.
Biorepositories may be needed for retaining samples, with data capture in an inventory management system.
Sponsors may deploy technologies that integrate with any or all of the data streams above. Most often, however, the first time a sponsor has visibility into the long chain of data related to clinical samples is upon arrival at the central lab.
Managing the multitude of disconnected technologies involved in conducting a biomarker-driven clinical trial requires expertise, and central lab services play a critical role in ensuring seamless execution and access to critical data.
Challenges for Central Labs
In this era of precision medicine, an increasing number of early phase clinical trials are leveraging biomarkers, both as part of study design and to inform development efforts. It has become more common to see complex clinical protocols with pharmacokinetic endpoints, immunogenicity or immune monitoring endpoints, and multiple biomarker endpoints. Each of these endpoints may require an assay run at a different lab, resulting in studies involving half a dozen or more specialty labs.
With so many moving parts, central labs must always know the location, condition, and status of every sample and how to retrieve them throughout their life cycle. Central labs often need to manage:
- Multiple couriers (if specialty regional access is required)
- Supply and kit inventory
- Sample processing quality, ensuring consistency across labs
- Multiple screening and specialty labs and biorepositories that may not be regionally located, centrally managed, or harmonized
- Multiple technologies for managing source data
Streamlining Sample Management
Integrating as many services as possible from a single provider can help streamline the supply chain and reduce sources of risk, delays, and costs. To manage all these complex movements, that single provider would need:
- Clinical trial expertise
- Courier management experience
- Longstanding international and domestic industry relationships
- Global, integrated networks of depots and storage facilities
- Knowledge of customs regulations and regulatory agency guidelines
Most importantly, that single provider would operate in accordance with a deep understanding of the importance of managing all clinical trial materials, equipment, and supplies under appropriate conditions and delivering them to the right location at the right time to support study success.
The Emergence of Translational Central Lab Services
The ideal solution for biomarker-driven clinical trials is a translational central lab service, which exists at the nexus of translational science, clinical trial operations, and technology. Translational science informs biomarker and sample selection which, in turn, influences clinical trial design, sample collection, assay selection, and sample management methodology. At Precision for Medicine, we offer comprehensive translational central lab services solutions for precision medicine trials:
Clinical sample kitting and logistics
Consistent collection and management of clinical samples is critical for sample quality. We provide all aspects of clinical kitting, from custom kit development and production to clinical site training, global shipping and logistics, and supply chain management, all tailored to the specific sampling and processing needs of each study.

Precision Starts Here—Don’t Let Sample Errors Derail Your Trial
Learn how our expert-driven kitting ensures 100% compliance
Clinical sample processing
Precision offers real-time sample processing, with sample processing labs on 5 continents providing the full range of PBMC, blood, and tissue processing services. This includes same- or next-day PBMC isolations.
Biospecimen management and biorepository
Appropriate sample handling is critical for assay quality. Precision provides inbound and outbound sample management for all sample types, with full chain of custody and sample accessioning. We have experience with all major couriers and are well-versed in export and import requirements. Further, our secure 100,000 sq. ft. primary repository can store all sample types at controlled ambient temperatures, +4°C, -20°C, -80°C, and in liquid nitrogen. The main Biorepository hub sits in Frederick, MD with satellite locations across the US and EU/UK.
Biospecimen data
As study complexity increases, so does the chance for data errors or an inaccurate view of the status of clinical study samples. Supported by a dedicated Biospecimen Data Services team, Precision’s data management systems safeguard all aspects of sample data integrity and data quality through database parameters and data handling requirements that are customized to sponsor and study needs.
Precision Lab e-Portal
Round-the-clock access to critical study data is essential. Precision’s Lab e-Portal provides real-time visibility into project, kit, sample, and shipment status, allowing for enhanced management of central lab services activities.
With our global network of clinicians, screening and specialty labs, biorepositories, and logistics capabilities, we are a single-source solution for end-to-end sample and program management.
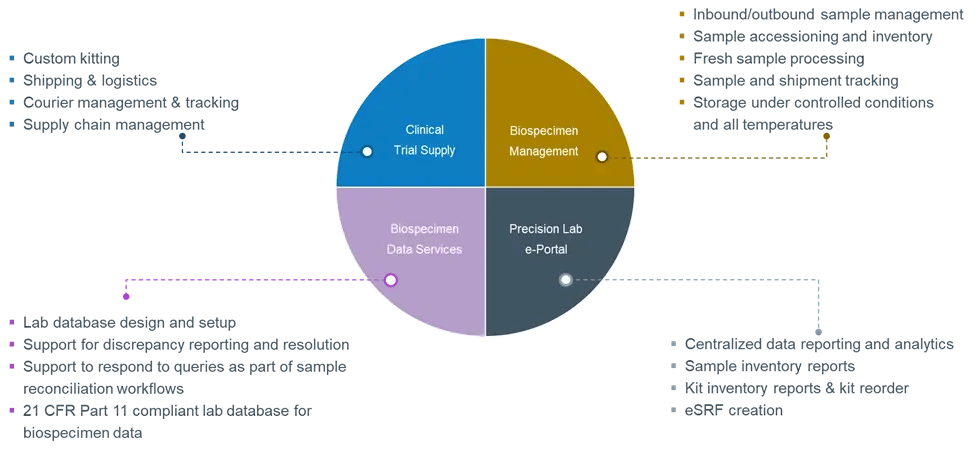 Figure 3. Precision for Medicine Translational Central Lab Services
Figure 3. Precision for Medicine Translational Central Lab Services
Precision for Medicine has also developed a virtual master sample inventory management system that offers centralized reporting via an intuitive user interface. This system provides dashboards with key performance indicators (KPIs) and data summaries by site, subject, and sample type, which allows sponsors and contract research organizations to identify patterns of noncompliance and focus on resolving discrepancies. Available on a web-based platform, this system enables more effective collaboration and allows for consolidation of all lab-related documentation in one place.
Precision for Medicine is purpose-built to accelerate therapeutic and diagnostic innovation through biomarker identification and assay development, clinical trial solutions, clinical trial sample protection, companion diagnostic development, and solutions that integrate all the disparate data generated during clinical trials.