The emergence of immuno-modulatory therapies has sparked increased interest in measuring cell-based receptor engagement and modulation in patients who have been treated with these novel medicines. Measuring receptor occupancy (RO) can be challenging and developing reproducible RO assays that can be used in clinical trials requires careful planning.
Importance of Reproducibility
When an assay is reproducible, the result obtained by one lab can reliably be replicated by other labs. Reproducibility is a fundamental requirement for assays used:
- In global clinical trials, where sample logistics are complex and multiple lab providers are used, reproducibility is essential
- For real-time assays, where biomarker stability may be an issue
- In long-term studies, where it is critical to be able to compare data acquired at the beginning of the study to that acquired at the end
Using a reproducible assay accommodates complex sample logistics, allows analysis of time-sensitive marker(s), and minimizes the risk of false data interpretation or loss of a signal in “noisy” data.
Reproducibility is achieved through a process called standardization, which focuses on eliminating any source of variability, from sample preparation and instrument setup to standard operating procedure (SOP) generation and operator training. With flow cytometry-based assays, standardization may be challenging for several reasons, including:
- The need to simultaneously measure multiple parameters that are interconnected
- The lack of readily available reference material to serve as an internal control
- The data to be analyzed often does not derive from a calibration curve, with the exception of receptor occupancy assays
- The process of data analysis requires highly trained and experienced analysts
Introduction to Receptor Occupancy Assays
RO is a flow cytometry-based pharmacodynamic (PD) that quantifies the binding of a drug to a specific target on the cell surface. Often, RO needs to be tested on fresh whole blood samples because over time ex-vivo, the receptor may be downregulated/internalized or the target cells may undergo apoptosis. Thus, when developing an RO assay, it is important to clarify the stability of receptor occupancy and to identify labs in close proximity to the sites where samples are collected unless stabilization of the signal is possible.
RO assays need to be customized to the drug of interest, so it is critical to define the best approaches for designing and developing these assays. There are three main formats that can be used to develop an RO assay:
- Free receptor assays measure the proportion of the receptor that is not bound by the drug. This can be done by using a fluorochrome-conjugated antibody that competes with the drug for the same epitope. Typically, this format is used for determining dose.
- Total receptor assays measure both free receptor and total receptor present on the cells in the sample. These assays utilize two antibodies—one that competes with the drug and another that recognizes a different epitope of the receptor. This format is useful when the drug is expected to affect up- or down-regulation of the receptor at a certain concentration. This requires that a non-competing antibody exists.
- Direct assessment of bound receptor assays are useful when the receptor expression is low or when the cells to be targeted are rare. In this format, the antibody binds directly to the drug, enhancing detection of drug that is bound to the receptor and increasing assay sensitivity.
The optimal format will depend on the aim of the study, the biology of the receptor, and the type of drug and perhaps most crucially what reagents are available or can be derived.
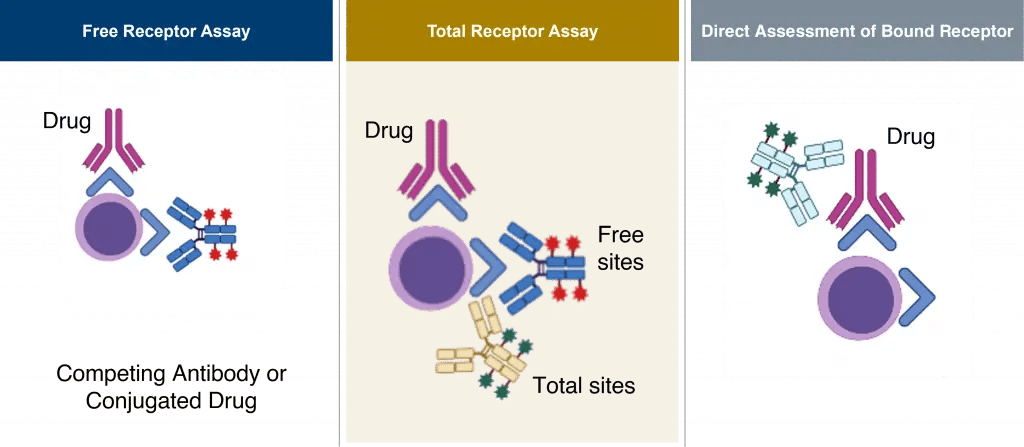 Figure 1. Receptor Occupancy (RO) Assay Formats
Figure 1. Receptor Occupancy (RO) Assay FormatsDesigning a Receptor Occupancy Assay
Careful assay design and reagent selection is crucial for the development of a reproducible RO assay that can be transferred from one lab to another. When designing an RO assay, it is critical to:
- Determine if the target receptor is abundant or expression is low
- Test different clones and fluorochromes to ensure they do not compete with the drug
- Test for any interference between the detector antibody and the drug
- Create a calibration curve showing saturating conditions of the drug, which will be used to quantify RO in clinical samples
Developing an RO Assay
Transitioning from design to development of an RO assay, it is essential to develop a comprehensive understanding of the biology of the RO. Mechanism of action, target cell population, receptor expression, and specimen stability should all be analyzed when performing in vitro testing of drug activity. All these factors contribute to the reliability of the calibration curve.
For flow cytometry-based assays, including appropriate controls is paramount. Marker expression, measured as mean fluorescent intensity (MFI), can be determined by tracking the antibody and ensuring that all the markers to be detected during flow cytometry analysis are saturated. The compensation matrix and the acceptable level of spillover can be set by standardizing the voltage of the instrument. The gating strategy, which defines positive or negative populations for a specific marker, can be determined using isotype or fluorescence minus one (FMO) controls. Finally, it is important to conduct experimental controls that ensure the assay performs as expected for the defined characteristics of the clinical sample or testing conditions.
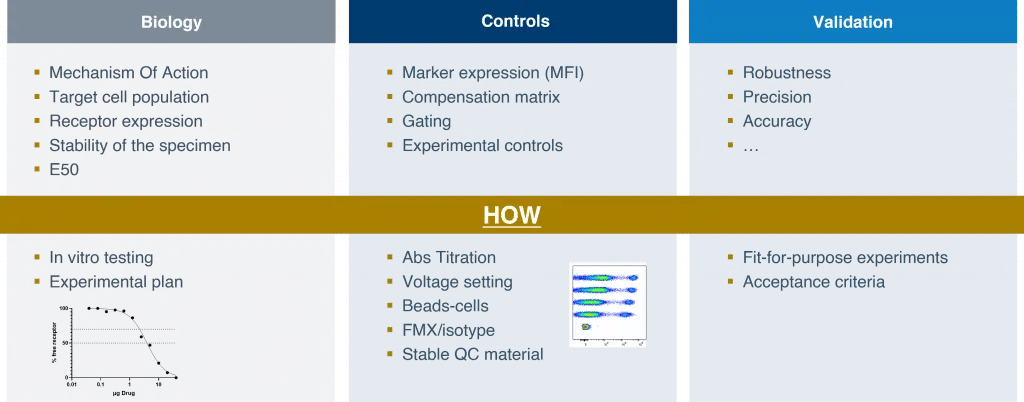 Figure 2. Key considerations for RO assay development and validation
Figure 2. Key considerations for RO assay development and validationValidating an RO assay
Once an RO assay has been developed, it needs to be validated. The validation process—along with the parameters and acceptance criteria—should be customized to the intent of the study. It is important to consider the study phase, the endpoint, and how the data will be reported. Flow cytometry assays are rarely used for a primary endpoint. For exploratory and secondary endpoints, Precision for Medicine has developed general guidelines on method validation for clinical studies, based on the intent of the data and with a focus on robustness (see Figure 3).
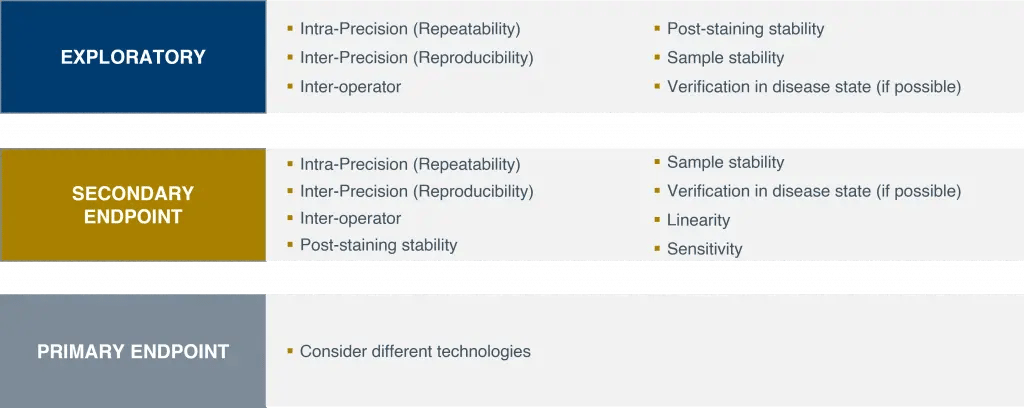 Figure 3. Guidelines on validating RO assays for clinical studies
Figure 3. Guidelines on validating RO assays for clinical studies
For real-time assays, we generally recommend training at least three operators to ensure full coverage over the life of a study. Validating post-staining and sample stability is important for clarifying the test window and time sensitivity of the assay. Verifying the assay in the disease state is also useful for understanding the true frequency of the cells in the expected clinical study population. For RO assays that will be used as a secondary endpoint, method validation will need to be more rigorous because the data acquired will be reported to regulatory agencies. In such cases, validation may also include linearity and sensitivity to clarify the performance of the assay and increase confidence in the clinical data.
Transferring an RO assay
Assay transfer is most seamless when each lab involved in the clinical study uses the same flow cytometry machine with the same configuration, as the reference values measured by the lab that developed and validated the assay can be transferred across all labs. What will be needed for the transfer are:
- Reference (CS&T) beads
- MFI values
- An SOP detailing all the steps of the assay process
Utilizing reagents from the same lots may also be useful. If this is not possible, a bridging study will need to be run to minimize lot-to-lot variability. For RO assays, it will be necessary to test at least three different concentrations of the drug to validate reproducibility within the defined acceptance criteria. To complete assay validation and transfer, it will also be important to train multiple operators and test multiple samples to further minimize variability.
Key takeaways
The ability to simultaneously evaluate receptor expression and drug occupancy on specific cell subsets is critical throughout the development of immuno-modulatory therapies. Developing, optimizing, and validating a robust, reproducible RO assay facilitates dose selection, safety, and pharmacological monitoring in clinical studies, supporting development of safe, effective therapeutics.
Discover how Precision for Medicine supports researchers in obtaining therapeutic insights through receptor occupancy assays by flow cytometry.