

Centralized Clinical Sample Data via the Precision Lab e-Portal
With the Precision Lab e-Portal, investigators can see clinical sample collection kit inventory and shipment status, and view clinical sample biostorage inventory reports.

Real-time management of clinical trial supplies and biospecimens
Powered by Precision Translational Sciences development team designed for management of complex datasets, the Lab e-Portal provides:
- Centralized inventory and kit data reporting
- On-demand data reporting
- Sample inventory reports
- Kit inventory reports
With the Precision Lab e-Portal you can:
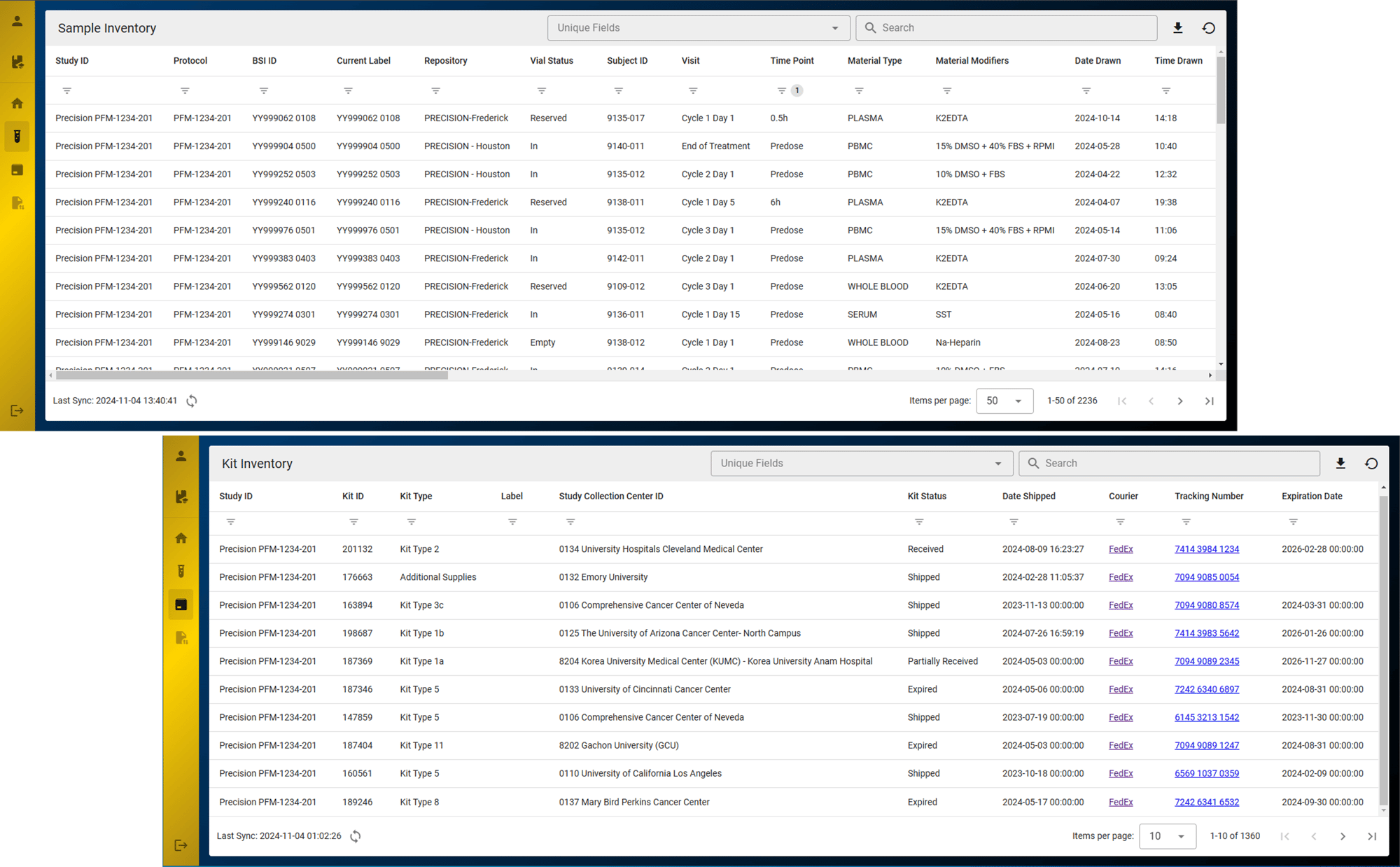
Instantly access your clinical sample and kit inventory levels
With just a few clicks, you can get a quick-glance summary of your clinical samples and kit statuses, resulting in greater visibility and improving study compliance.

Track your most essential samples
In addition to kitting inventory and expiry data reports, which can help prompt kit reorders, shipping data can be accessed, providing enhanced visibility into clinical kit status to ensure clinical sites are always well supplied.
Be confident in your sample data integrity
The accuracy of data in the Precision Lab e-Portal is assured by a team dedicated exclusively to global biospecimen data services. Because of the complexity of data biomarker-heavy clinical trials, a group that can customize processes to the needs of each trial is required.
Related Services
-
Explore


PBMC & Sample Processing
ExploreSample processing with expertise in PBMC isolations from labs across 5 continents

PBMC & Sample Processing
ExploreSample processing with expertise in PBMC isolations from labs across 5 continents
-
Explore


Biospecimens Data Services
ExplorePurpose-built data systems for specimen management, lab database design, and data reconciliation to ensure data integrity
Biospecimens Data Services
ExplorePurpose-built data systems for specimen management, lab database design, and data reconciliation to ensure data integrity -
Explore


Biostorage
ExploreSample storage under all conditions and temperatures, rapid sample accessioning, and online inventory access

Biostorage
ExploreSample storage under all conditions and temperatures, rapid sample accessioning, and online inventory access
