.jpg?width=1440&height=672&name=iStock-1256323051%20(1).jpg)
.jpg?width=850&height=1440&name=iStock-1256323051%20(1).jpg)
Specialty Labs, Central Lab Services, and Biomarker Solutions
.jpg?width=2000&name=iStock-1181391587%20(1).jpg)
Precision is custom built to address the complexity of modern therapeutic and diagnostic development
With expertise in today’s most advanced techniques for deeply interrogating samples to better understand patient biology, at scale, Precision can support even the most challenging global development programs.
Turning scientific challenges into solutions through a holistic approach to translational and biomarker services
Our solutions begin with biospecimens, through a spectrum of laboratory sciences from biomarker identification to clinical immune monitoring. We provide complex central lab services including custom kitting and global logistics, and have a regulatory team dedicated to the needs of companion diagnostics and diagnostics. Throughout, we coordinate to always provide personalized services to best support the needs of your program.
-
Explore
Specialty Lab Services
ExploreSolutions for preclinical thorough clinical development across a broad range of sample types, modalities, and analytical techniques.
Leveraging established and proprietary technologies, we develop and validate biomarker assays with a consultative, customized approach.
-
Explore
Central Lab Services
ExploreBiomarker-driven and precision medicine trials have unique complexities in kit development, logistics, sample management, and data collection.
Precision's robust solution to central lab services is rooted in a deep understanding of the needs of both early-phase trials and those with complex biomarker designs.
-
Explore
Companion Diagnostics
ExplorePrecision for Medicine’s IVD Regulatory Team provides full lifecycle regulatory support to ensure commercialization success and beyond. Expert guidance is essential for the challenging journey of bringing a diagnostic or CDx from development to market.
-
Explore
Biospecimens
ExplorePrecision for Medicine has built one of the world’s most extensive biobanks and sample collection networks, capable of meeting the stringent needs of any disease research program.
In addition to biorepositories containing over 10 million specimens, Precision has an in-house donor center and processing lab to generate and ship custom samples rapidly, and a network of hospitals and clinical care centers to collect samples in virtually any disease indication.
-
Explore
Solutions to Advance Science
ExploreAt Precision, we are dedicated to pioneering advancements that empower researchers with precise, actionable insights. This includes developing technologies that we offer as services, including ApoStream® and Epiontis ID®, which provide sophisticated solutions for circulating tumor cell liquid biopsy analysis and immune cell profiling.
Precision’s specialty lab approaches include a full spectrum of biomarker and sample analysis techniques
Experience and platforms to deeply interrogate any sample or tissue type
We leverage proprietary and established technologies, develop and validate biomarker assays needed to generate robust biomarker data in samples ranging from nucleic acids to tissues.
-
DNA
DNA
-
PCR—ddPCR, qPCR
-
Immunophenotyping via proprietary epigenetic platform
-
Sequencing—whole genome, whole exome, targeted sequencing
-
cfDNA analysis
-
-
RNA
RNA
-
Gene expression profiling—NanoString
-
Bulk and single-cell RNASeq
-
RT-PCR and RT-ddPCR
-
Spatial transcriptomics
-
-
Protein
Protein
-
Comprehensive large-molecule bioanalysis—PK, ADA, NAb, TAb
-
Multiplex cytokine profiling, receptor occupancy, tetramer staining
-
Custom ligand binding assays—ELISA, MSD, Olink®
-
Quantitative image analysis of protein expression (eg, phosphorylation, signaling)
-
Automated Western blotting—Jess™
-
-
Cell
Cell
-
Flow cytometry—standard and spectral flow up to 64 color panels
-
Functional assays—eg, ELISpot/FluoroSpot, T-cell activation, ADCC
-
Single-cell quantitative image analysis
-
Proprietary cell separation technology for CTCs
-
-
Tissue
Tissue
- Multiplex IHC with centralized pathology reading
- Quantitative IF—up to 9 concurrent markers
- FISH, ISH, sequencing
- GLP tissue cross-reactivity (TCR)
Discover how our translational and biomarker sciences can advance your preclinical or clinical development program
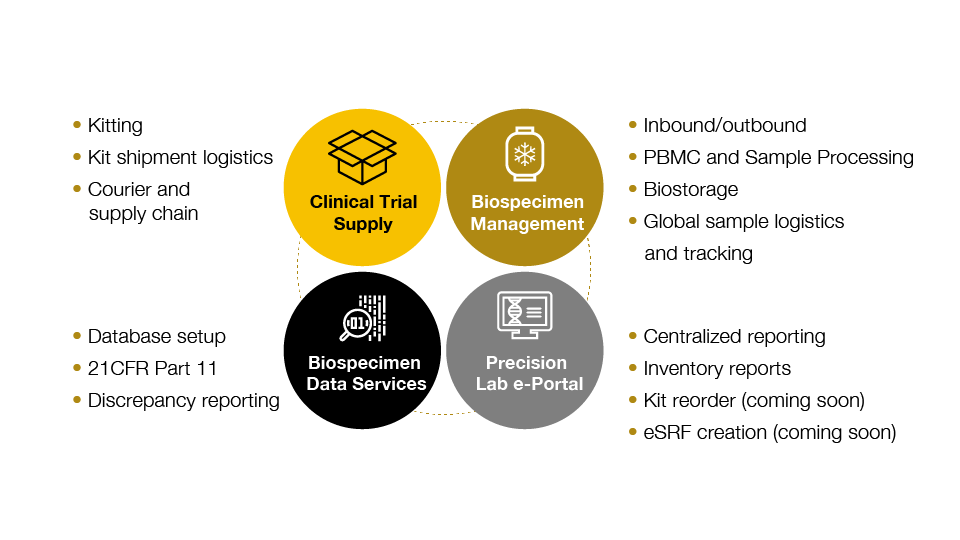
Precision’s central lab services are designed for biomarker-driven clinical trials
Through a deep understanding of what is needed to develop and run successful specialty assays, Precision understands the importance of the quality of clinical samples, and what is needed to ensure that quality - including how to design the most appropriate sample collection protocols and collection kits.
Our highly customized central lab services support global trials including kitting, logistics, sample processing, biostorage, and related biospecimen data services.
Precision’s harmonized approach to sample collection, processing, logistics, storage, and reporting

Our companion diagnostics development and IVD regulatory team provides full life-cycle support
Bringing a product from development to market can be challenging to navigate, which is why it is crucial to have a team of experts who know the ins and outs of the development life cycle for your diagnostic or companion diagnostic. Precision has experience in developing clinical trial assays, and our team of in vitro diagnostic regulatory affairs experts can shepherd the regulatory process, ready to walk with you step-by-step to streamline the path to success for your product.
Enabling research with an expansive biospecimens repository and custom collections
Precision’s biorepositories contain millions of biospecimens available to researchers, including both healthy and disease-state tissue and liquid biospecimens. We also routinely perform custom collections through both our in-house donor center and through a network of collection sites.
Below are some of the categories of biospecimens Precision has available.
Advancing science with novel solutions for liquid biopsy and immune monitoring
In addition to excellence in utilizing existing assay and biomarker platforms, we have developed our own biomarker solutions in key areas to provide greater insights.
Advancing the science of biomarker-driven research is just one part of our overall commitment to being at the forefront of precision medicine and precision therapeutic development.

ApoStream®—Novel approach to rare cell enrichment and CTC liquid biopsy
Precision’s complete solution for CTC liquid biopsy is enabled via ApoStream®, a device that isolates and enriches CTCs, facilitating any type of downstream analysis, such as multiplex quantitative immunofluorescence or FISH/ISH.
ApoStream® can also be used to collect other rare cell types, such as stem, progenitor, and differentiated immune cells, including CAR T cells and other difficult-to-identify immune cell populations for immuno-oncology applications.
Epiontis ID®—Unique approach to immune monitoring
Precision’s Epiontis ID® technology, with its unique approach to cell phenotyping, is a robust, adaptable, and cost-effective solution for immune cell profiling. Epiontis ID® uses unique methylation patterns to distinguish cell types, as all cells have the same DNA but differ in gene expression. This approach amplifies demethylated DNA regions in specific cells using a custom process and qPCR primers, allowing precise cell count in any sample.