Updated October 2025
The State of Antibody-Drug Conjugate Clinical Trials in 2025
Antibody–drug conjugates (ADCs) are a rapidly expanding class of targeted cancer therapies that combine the specificity of monoclonal antibodies with the potency of cytotoxic agents. These constructs are designed to deliver chemotherapy directly to tumor cells while minimizing damage to healthy tissue. As of 2024, more than 100 ADCs are in clinical development, with 20 FDA-approved indications across hematologic and solid tumors.1
The clinical trial landscape for ADCs is evolving quickly. Sponsors are exploring new payload mechanisms, including topoisomerase inhibitors, DNA alkylators, and microtubule disruptors. Trials are also investigating novel linkers and conjugation technologies to improve stability, reduce off-target toxicity, and enhance tumor penetration. HER2, TROP2, and BCMA remain dominant targets, but newer ADCs are being designed for HER3, CEACAM5, and Nectin-4, expanding the therapeutic reach.1
Dual-payload ADCs are entering early-phase trials, aiming to overcome resistance and tumor heterogeneity by delivering multiple cytotoxins simultaneously. These designs require careful dose optimization and safety monitoring, especially in solid tumors where antigen expression can vary widely. Biomarker-driven enrollment and adaptive trial designs are helping sponsors manage complexity and improve response rates.2
Geographically, ADC trials are concentrated in North America, Europe, and East Asia, with increasing interest in decentralized models to improve access. The regulatory environment is also adapting, with agencies offering expedited pathways for ADCs that demonstrate strong early efficacy and manageable safety profiles.
This article reviews the current ADC trial landscape, including trial status, geographic distribution, patient segments, and therapeutic focus. It also outlines how Precision for Medicine supports sponsors in executing ADC trials with technical precision and operational discipline.
Where ADC Trials Stand Today: A Pipeline Overview
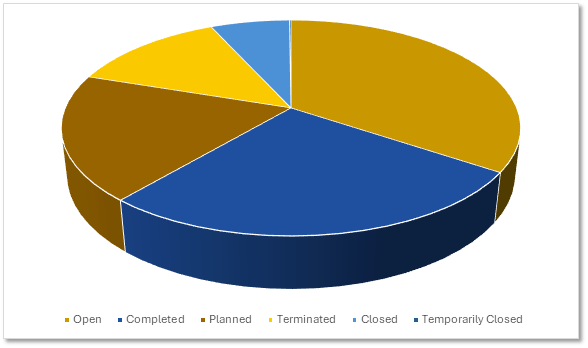
The ADC trial pipeline spans all phases of development indicating sustained investment in clinical validation. Sponsors are balancing risk with opportunity in a competitive space.
ADC Trials by Development Status
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
ADC Trial Activity: Five-Year Growth Trajectory
ADC trial starts have accelerated in recent years mirroring broader oncology trends in targeted therapeutics.
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
Global Footprint: Where ADC Trials Are Happening
ADC trials are geographically concentrated in China and the U.S., followed by Europe, Australia and East Asia.
Planned & Ongoing ADC Trials by Site Country
|
Countries |
Count |
|
China |
812 |
|
United States |
524 |
|
Spain |
233 |
|
Australia |
201 |
|
South Korea |
197 |
|
France |
196 |
|
Italy |
176 |
|
Japan |
175 |
|
United Kingdom |
168 |
|
Canada |
167 |
Citeline Trialtrove® – 07OCT2025
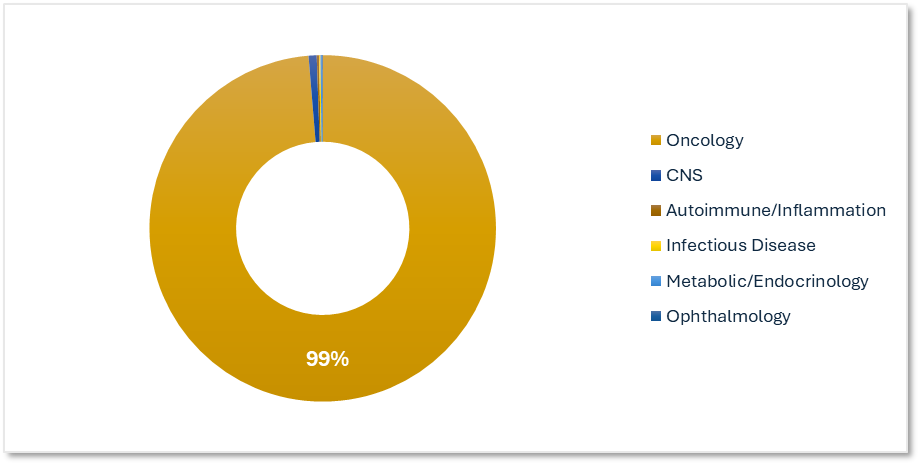
Therapeutic Focus: Cancer Types Leading ADC Development
Therapeutic area trends show a clear emphasis on cancer indications where targeted delivery offers clear advantages. This focus aligns with ADCs' mechanism of action and clinical promise.
Planned & Ongoing ADC Trials by Therapeutic Area
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
Top Cancer Indications in ADC Clinical Research
ADC trials are most common in breast, lung, and esophageal cancers.
Planned & Ongoing ADC Trials by Indication
|
Indication |
Count |
|
Unspecified Solid Tumor |
515 |
|
Breast |
339 |
|
Lung, Non-Small Cell |
327 |
|
Esophageal |
231 |
|
Gastric |
221 |
|
Ovarian |
180 |
|
Colorectal |
140 |
|
Bladder |
139 |
|
Head/Neck |
135 |
|
Pancreas |
121 |
Citeline Trialtrove® – 07OCT2025
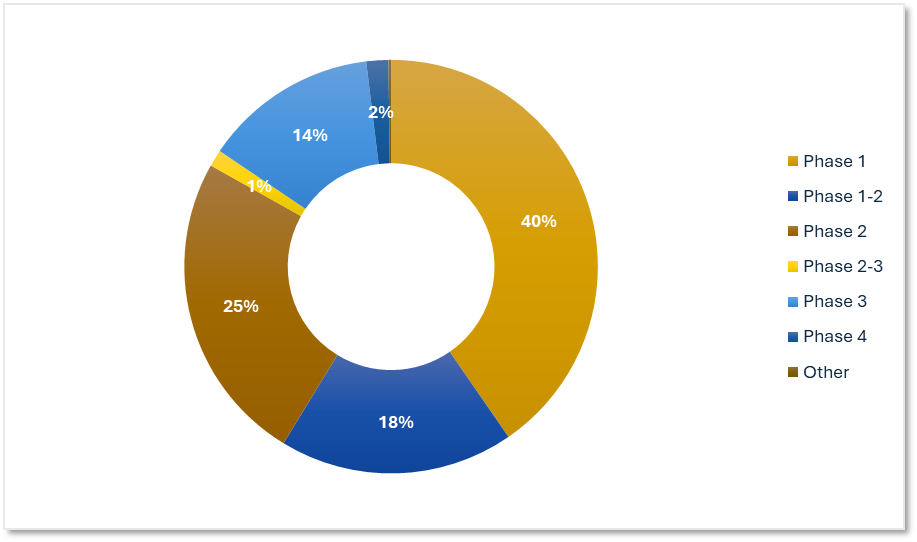
Early-Stage Development Dominates ADC Trial Activity
Phase data shows a strong emphasis on early development highlighting the exploratory nature of ADC research. Late-phase trials may increase as early data matures.
Precision for Medicine's ADC Trials by Phase
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
How Precision Drives ADC Clinical Research
Our ADC Trial Portfolio: Growing with the Market
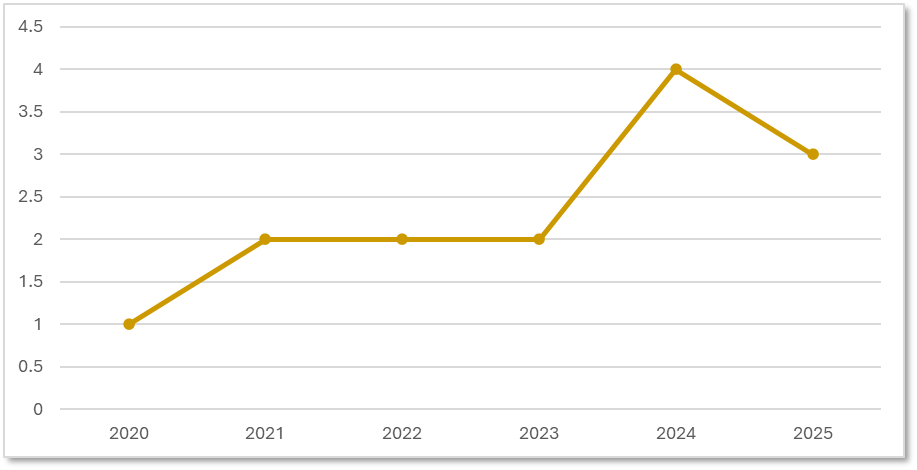
Precision for Medicine has initiated multiple ADC trials, focusing on the last 5 years we have seen an upward trend, which aligns with overall global trends in targeted oncology. Due to our diverse experience, our study teams are equipped to manage complex ADC protocols, spanning multiple indications.
Precision for Medicine's ADC Trials by Start Date
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
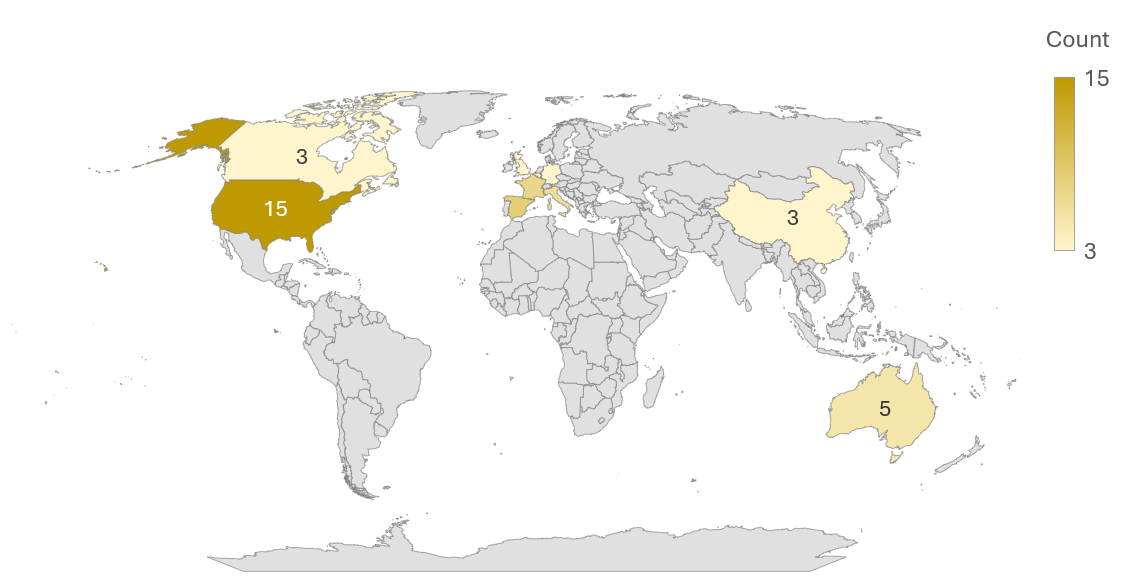
Global Capabilities: Precision's ADC Trial Network
Our geographic footprint includes North America, Europe, and Asia. When it comes to ADC trial execution, Precision adapts to regional nuances.
Precision for Medicine's ADC Trials by Site Country
Citeline Trialtrove® – 07OCT2025
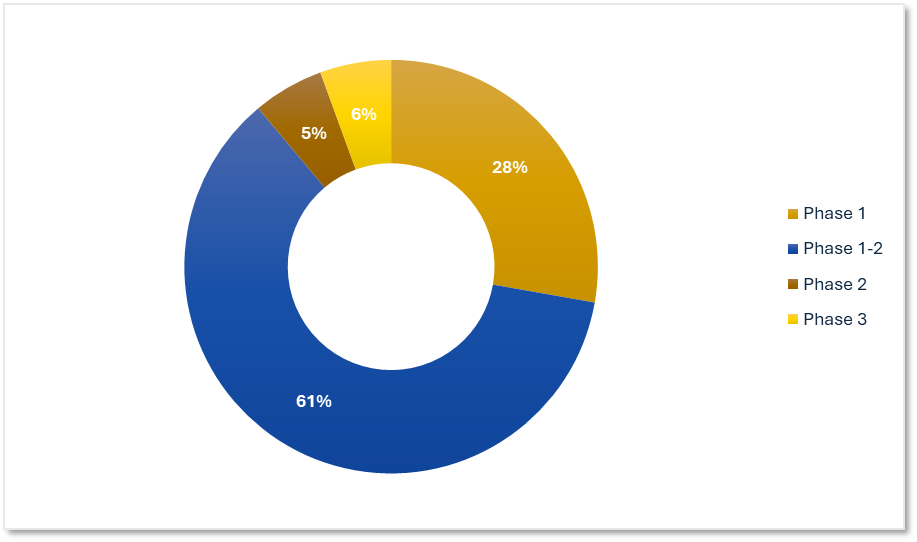
Precision's ADC Trial Experience by Phase
Our work spans early discovery through late-stage validation with a strong presence in Phase 1 and Phase 1-2. Our teams successfully manage dose escalation, biomarker integration, and close safety oversight.
Precision for Medicine's ADC Trials by Phase
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
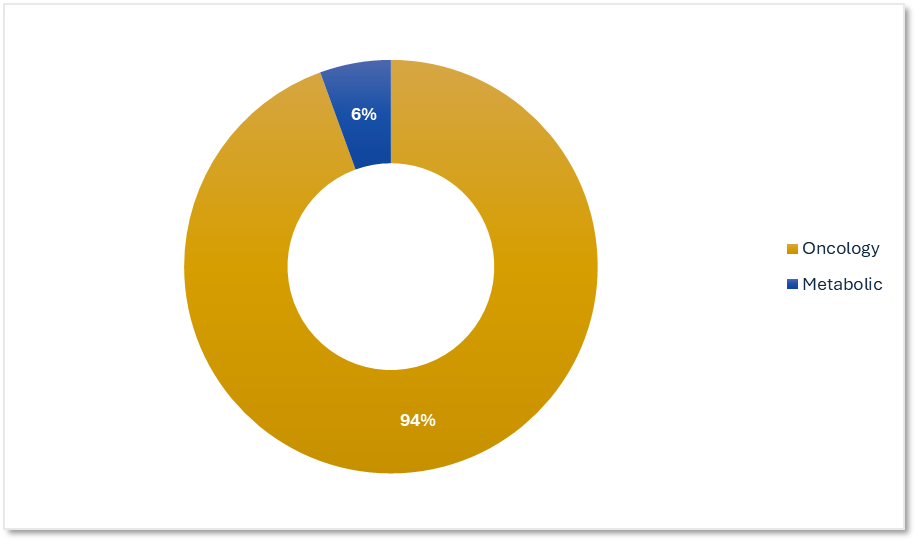
ADC Therapeutic Specialization from an Oncology-focused Clinical Research Organization
Our ADC trials are focused on oncology indications with complex dosing and safety profiles. We help sponsors optimize therapeutic targeting.
Precision for Medicine's ADC Trials by Therapeutic Area
 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
Target Patient Populations: Precision's ADC Indication Expertise
Our portfolio includes studies with the following participant populations, our top indications are NSCLC, Pancreas, Ovarian, and Breast Cancer. With our broad experience, Precision study teams can help sponsors navigate indication-specific challenges.
Precision for Medicine's ADC Trials by Top Indications
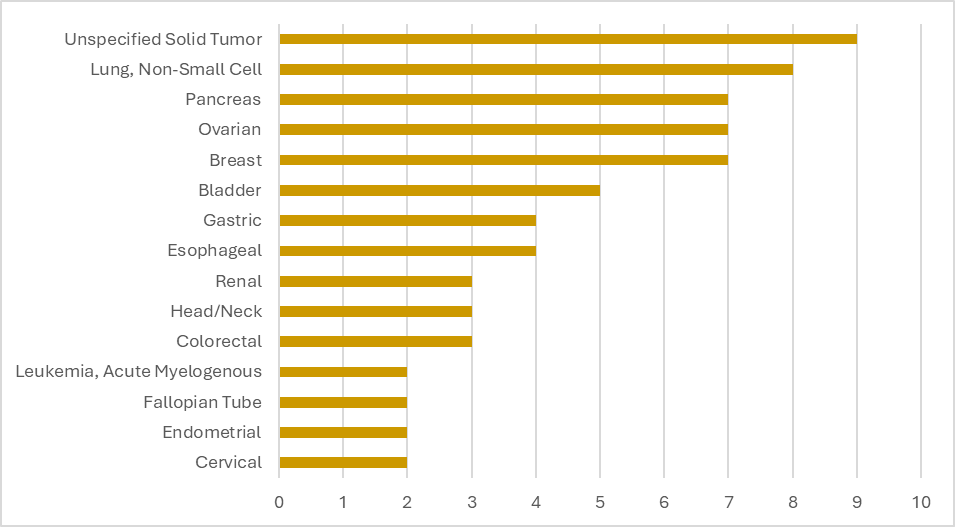 Citeline Trialtrove® – 07OCT2025
Citeline Trialtrove® – 07OCT2025
Partner with Precision for Your Upcoming ADC Clinical Trial
ADC trials require specialized planning and execution, particularly when managing complex dosing schedules and safety oversight. Precision for Medicine works as a partner with our sponsors to operationalize these studies efficiently. From our Precision Site Network (PSN), supporting with feasibility & site selection, to our integrated data management model providing fast data review, our teams bring experience and solution focused study management strategies helping ensure ADC trials are delivered with consistency and clarity across global geographies.
When your ADC program demands both speed and scientific rigor,
Precision for Medicine delivers the expertise to get it right the first time.
-
Explore

Oncology CRO Services
ExploreTomorrow's targeted therapies require a shift away from yesterday's processes. Precision's specialized oncology CRO has integrated and aligned capabilities to efficiently navigate and execute complex clinical trials.Oncology CRO Services
ExploreTomorrow's targeted therapies require a shift away from yesterday's processes. Precision's specialized oncology CRO has integrated and aligned capabilities to efficiently navigate and execute complex clinical trials. -
Explore

Fast Study Startup
ExplorePrecision's approach to Study Start Up combines technological innovation, regulatory insight, and global expertise to optimize every phase of trial initiation, ensuring your study begins with the momentum needed to succeed.Fast Study Startup
ExplorePrecision's approach to Study Start Up combines technological innovation, regulatory insight, and global expertise to optimize every phase of trial initiation, ensuring your study begins with the momentum needed to succeed. -
Explore

Speak with an Expert
ExploreReady to meet Precision specialists? Tell us about your program and we'll get back to you right away.
Speak with an Expert
ExploreReady to meet Precision specialists? Tell us about your program and we'll get back to you right away.
Frequently Asked Questions
How many ADC clinical trials are currently active globally?
As of October 2025, there are over 100 antibody-drug conjugates in clinical development worldwide, with planned and ongoing trials concentrated primarily in China (812 trials) and the United States (524 trials). The pipeline spans all development phases, with a strong emphasis on early-phase research as sponsors explore novel payload mechanisms, linker technologies, and targeting strategies. This sustained investment reflects the growing confidence in ADCs as a transformative approach to targeted cancer therapy.
What makes ADC trials operationally complex compared to traditional oncology studies?
ADC trials require specialized expertise due to complex dosing schedules, dose escalation protocols, and intensive safety monitoring for both on-target and off-target toxicities. Dual-payload ADCs add additional complexity by delivering multiple cytotoxins simultaneously, requiring careful optimization to manage tumor heterogeneity and resistance. Biomarker-driven enrollment, adaptive trial designs, and the need for regional regulatory expertise across multiple geographies make operational precision critical. Sponsors benefit from partners experienced in Phase 1 and Phase 1-2 trials who can manage these technical challenges while maintaining data quality and patient safety.
What are the most common cancer indications for ADC clinical trials?
ADC trials are most prevalent in solid tumors, with unspecified solid tumors (515 trials), breast cancer (339 trials), and non-small cell lung cancer (327 trials) leading the landscape. Other significant indications include esophageal (231 trials), gastric (221 trials), and ovarian cancers (180 trials). This distribution reflects both the clinical promise of targeted delivery in these malignancies and the availability of well-characterized tumor antigens like HER2, TROP2, and emerging targets such as HER3 and Nectin-4.
Which countries should I prioritize for global ADC trial site selection?
Geographic strategy should balance trial volume, regulatory environment, and enrollment capacity. China leads with 812 active trial sites, followed by the United States (524), making these two markets essential for competitive recruitment timelines. Europe offers strong infrastructure, with Spain (233), France (196), Italy (176), and the UK (168) providing robust access to patients and established regulatory pathways. Australia (201) and South Korea (197) are emerging as strategic locations for early-phase trials. A multi-regional approach spanning North America, Europe, and East Asia optimizes access to diverse patient populations while managing regulatory and operational complexity.
References
- Flynn P, Suryaprakash S, Grossman D, Panier V, Wu J. The antibody–drug conjugate landscape. Nat Rev Drug Discov. 2024;23(8):577-578. doi:10.1038/d41573-024-00064-w
- Mullard A. Dual-payload ADCs move into first oncology clinical trials. Nat Rev Drug Discov. 2025;24:573-576. doi:10.1038/d41573-025-00121-y