Tissue Biopsy vs Liquid Biopsy
While tissue biopsy remains the gold standard for disease diagnosis and research, interest in liquid biopsy as an alternative or adjunct to tissue sampling is increasing. Presently, experts are actively exploring liquid biopsy in precision medicine for cancer detection, screening for disease recurrence, evaluating treatment response, and assessing residual disease at the molecular levels.
With tissue biopsy, nucleic acids and proteins can be assessed in situ, along with their architectural and spatial context. However, tissue sampling is invasive, not amenable to repetition and might be technically challenging or associated with unacceptable procedural risks. Further, in clinical studies, a requirement for multiple biopsies significantly slows down enrollment rate due to high patient burden. When tumor tissue sampling is not feasible, liquid biopsy offers a minimally invasive alternative. Liquid biopsy also enables serial testing across multiple timepoints, which could be used for longitudinal monitoring of whole-body disease burden, tumor progression or therapeutic response.
Cancer, as a spatially and temporally dynamic disease, demonstrates heterogeneity not just within tumors, but also between primary and metastatic tumors in the context of precision medicine. Consequently, single tissue biopsies may lead to drastic underestimation of the full genomics landscape of an individual patient’s cancer.[1] Thus, liquid biopsy can serve to complement traditional tissue-based assays, providing additional or adjunctive insights.
In this article, we investigate the two main genomics-based methods in liquid biopsy: analysis of circulating DNA/RNA and circulating tumor cells (CTCs), examining their advantages, drawbacks, and possible applications in precision medicine.
Circulating DNA in Liquid Biopsy
Certain liquid biopsy techniques in precision medicine examine circulating nucleic acids, encompassing both DNA and RNA. Circulating nucleic acids comprise both circulating tumor DNA and RNA (ctDNA and ctRNA) and cell-free DNA and RNA (cfDNA and ctRNA). Studies have revealed that ctDNA, or tumor-derived DNA fragments unassociated with cells, accurately represents the tumor’s origin, making it valuable in liquid biopsy for precision medicine. A study comparing targeted sequencing of ctDNA and matched tissue biopsies from patients with metastatic castration-resistant prostate cancer (mCRPC) showed high concordance in the gene alterations identified.[2]
Meanwhile, cfDNA and cfRNA describe any nucleic acids that are circulating in the bloodstream but are not necessarily of tumor origin. Cancer patients typically exhibit higher average cfDNA levels compared to healthy individuals, and numerous studies in liquid biopsy research have shown a strong correlation between mutations identified in cfDNA and those discovered in tissue biopsy samples.[3] There is also evidence that levels of plasma cfDNA increase as tumors progress and that cfDNA acts as a signaling molecule to induce metastasis.[4] In addition, recent research focused on cfDNA methylation signatures and fragmentation patterns demonstrates promise for screening, early detection, and monitoring of cancer. Moreover, accumulating knowledge indicates that non-coding circulating RNAs play a critical role in the pathogenesis of various cancers and that dysregulated microRNA expression levels may be useful as clinical biomarkers.[5]
Genomic profiling of circulating DNA is generally performed using digital droplet PCR (ddPCR) if the researchers has identified a gene mutation of interest or NGS if the mutation of interest has not yet been delineated. Sequencing of ctDNA generates a large volume of information, including the mutation status of dozens or hundreds of oncogenes simultaneously. However, key limitations of ctDNA are that it does not provide any information on the expression levels of any given protein or any exon-skipping events or splice variants and that its clinical utility for primary cancer screening and minimal residual disease (MRD) monitoring remains unproven.[6]
Circulating Tumor Cells in Liquid Biopsy
In the last decade, advancements in isolating circulating tumor cells (CTCs) have enabled single-cell level analysis, allowing researchers to study spatial and temporal dynamics in circulation within liquid biopsy and precision medicine.[7] A growing body of evidence demonstrates that CTCs—cancer cells that have migrated into the bloodstream—undergo dynamic molecular changes in response to systemic therapy and may have clinical utility as functional biomarkers. In metastatic breast cancer, prevalence of CTCs in the blood has been correlated with lower progression-free and overall survival and has been shown to be of higher prognostic value than conventional imaging.[8],[9]
CTC-based liquid biopsies have the advantage of preserving cellular contents, allowing for gene expression profiling and other downstream analyses at the single cell level. In addition, tumors may harbor segregated clones, which could easily be missed by biopsy but may be captured by CTCs. Moreover, target biomarkers on the surface of CTCs can easily be identified with simple antibody binding, as long as the respective antibody is available.
Comparison of Circulating DNA and Circulating Tumors Cells[10]
| Advantages | Limitations | |
| Circulating DNA |
|
|
| Circulating Tumor Cells |
|
|
Applications of Liquid Biopsy
Genomics-based liquid biopsy offers extensive potential in various oncology applications, from deciphering a drug’s mechanism of action to categorizing patients and tracking treatment response in precision medicine. Currently, most of the FDA-approved liquid biopsy clinical diagnostics use ctDNA or cfDNA. In 2020, two companion diagnostics (CDx) that combine liquid biopsy and next-generation sequencing (NGS) were approved, demonstrating the feasibility and utility of targeting multiple genes to guide clinical decision-making.
Applications for Circulating DNA
cfDNA, extensively researched in various cancer types within precision medicine, shows potential as a tool for cancer detection, tumor mutation assessment, treatment eligibility determination, tumor dynamics and therapy response monitoring, and overall survival prediction.[12] In a secondary analysis of the BOLERO-2 clinical trial, prevalence of ESR1 mutations in cfDNA was inversely correlated with overall survival.[13] Numerous studies have demonstrated ctDNA can be used for minimal residual disease (MRD) detection and monitoring after treatment, which aids in assessing response, prognosis, and risk of recurrence.[14] Circulating DNA has also been explored extensively as a tool for providing early assessments of response to immune checkpoint inhibitor therapy.[15]
Applications for Circulating Tumor Cells
CTCs have a wide range of applications, from evaluating pharmacodynamic or mechanistic markers and establishing dosing strategies to characterizing heterogeneous CTC subpopulations and selecting patients for clinical trial enrollment. They can also be used as prognostic or predictive biomarkers. For example, in mCRPC, a high degree of phenotypic heterogeneity in CTCs is linked to decreased overall survival.[16] Further, in a small study of patients with pancreatic cancer, researchers found that a sufficient number of CTCs could be obtained from 10 ml of anticoagulated blood to allow for multiparameter phenotyping using qualitative immunofluorescence to identify features that could potentially serve as selective, predictive, or prognostic biomarkers.[17]
Interrogating CTC biomarkers may also be useful for predicting or monitoring treatment response. For example, in the BEACON trial, multiplex immunofluorescence (mIF) was performed on CTCs isolated from patients with metastatic breast cancer treated with etirinotecan pegol (EP) to measure expression of potential response biomarkers. It was found that topoisomerase (Top1) expression might be useful for identifying those patients who were most likely to experience an overall survival benefit with EP treatment.[18]
Another potential application of CTCs is in patient selection for clinical trials of solid tumor CAR-T cell therapy and other targeted therapeutics. Typically, patients would be stratified using immunohistochemistry (IHC), but if tissue biopsy is not possible, testing CTCs for the target biomarker would be an alternative approach to making enrollment decisions.
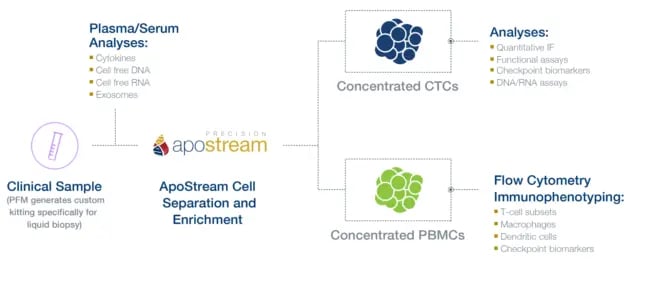
ApoStream: Advanced Technology for Isolating and Enriching Rare Cells
Circulating DNA and CTCs circulate at low frequencies. To generate clinically meaningful information, isolation and enrichment of the nucleic acid or cell of interest is generally required. ApoStream, Precision for Medicine’s proprietary CTC platform, uses a dielectrophoresis-based, antibody-independent separation approach to isolate and enrich CTCs. This technology can also be used to isolate other rare cell types such as stem cells, CAR-T cells, and other difficult-to-identify immune cell populations. With ApoStream, enriched cells remain intact and can be integrated with any downstream assay, including mIF, NGS, fluorescence in situ hybridization (FISH) and ISH (see Figure 1).
Conclusion
Liquid biopsy holds great potential for the diagnosis, treatment, and monitoring of patients with cancer. Circulating DNA and circulating tumor cells (CTCs) are both expected to have complementary functions as cancer biomarkers in precision medicine, potentially working together to inform clinical decisions. As technology continues to advance, the clinical applications of liquid biopsy in precision medicine will keep growing, extending beyond oncology to various therapeutic areas.
Discover how Precision for Medicine supports researchers in obtaining molecular insights through liquid biopsy in precision medicine.
References
- Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. NEJM 2012; 366: 883-92.
- Wyatt AW, et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017;109.
- Lebofsky R, et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol. 2015; 9:783-790.
- Bennett CW, Berchem G, Kim YJ, El-Khoury V. Cell-free DNA and next-generation sequencing in the service of personalized medicine for lung cancer. Oncotarget. 2016;7(43):71013-71035.
- Toden S, Goel A. Non-coding RNAs as liquid biopsy biomarkers in cancer. 2022;126(3):351-360.
- Dang DK, Park BH. Circulating tumor DNA: current challenges for clinical utility. 2022;132(12):e154941.
- Lim SB, et al. Liquid biopsy: one cell at a time. NPJ Precis Oncol. 2019;3:23.
- Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791.
- Giuliano M. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014;16(5):440.
- Lang JE. Advantages and disadvantages of ctDNA vs CTC assays: How to move the need forward towards clinical application. Available at https://cdp.cancer.gov/resources/technology_development_resources/ctdna/Lang.pdf.
- Lindsay C.R. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol. 2017;28(7):1523–1531.
- Qvick A, et al. Liquid biopsy as an option for predictive testing and prognosis in patients with lung cancer. Mol Med. 2021;27:68.
- Chardarlapaty S, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: A secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016;2(10):1310-1315.
- Peng Y, et al. Circulating tumor DNA and minimal residual disease (MRD) in solid tumors: Current horizons and future perspectives. Front Oncol. 2021;11:763790.
- Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022 Sep 12;15(1):131.
- Scher HI, et al. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res. 2017;77:5687-5698.
- Eisen A, et al. Abstract 428: Phenotyping pancreatic cancer CTCs as biomarkers for RX-3117 clinical trials. 2019. doi: 10.1158/1538-7445.AM2019-428.
- Rugo HS, et al. Change in topoisomerase 1-positive circulating tumor cells affects overall survival in patient with advanced breast cancer after treatment with etirinotecan pegol. Clin Cancer Res. 2018;24(14):3348-3357.




