Since the beginning of the SARS-CoV-2 pandemic, a key focus of research has been characterizing the human immune response in relation to the virus. Understanding how immune cell profiles change during acute infection or in response to vaccination may be key to predicting disease course or vaccine efficacy. Over the past decade, epigenetic-based cell counting has emerged as a complement or alternative to flow cytometry for immunophenotyping. This technique utilizes differences in the methylation status of specific gene loci to distinguish target immune cell types from other cell populations, and can be applied to fresh, frozen or paper-spotted dried blood and other bodily fluids or tissues.
In a recent study, Precision for Medicine collaborated with investigators from the University General Hospital of Valencia in Spain and University Hospitals of the Ruhr-University Bochum in Germany to explore whether epigenetic immune cell counting could advance the efficiency and quality of diagnostic and immune monitoring related to COVID-19. This study leveraged immune cell type-specific epigenetic assays originally developed for use in therapeutic clinical research in oncology and autoimmune disease. Given the availability of 35 existing assays for a wide range of key immune cell populations that are relevant in COVID-19 and the low sample requirements, our objective was to explore the use of epigenetic measurements in a pandemic setting.
Objectives for Non-Invasive Immune Monitoring
To evaluate the utility of epigenetic immune cell counting using Precision’s proprietary Epiontis ID technology for predicting COVID-19 disease course and assessing SARS-CoV-2 vaccine response.
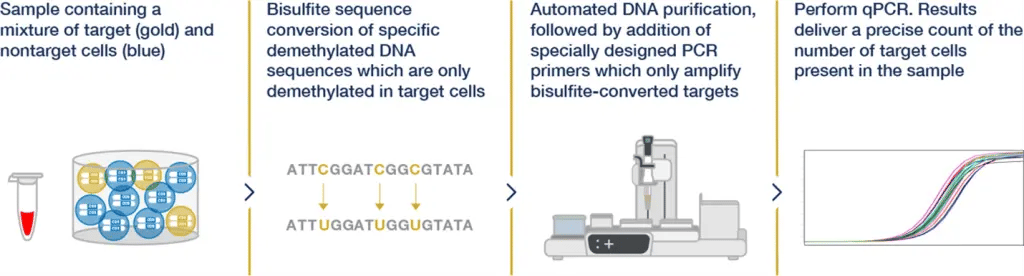 Figure 1. Process of Epigenetic Cell Counting
Figure 1. Process of Epigenetic Cell CountingPredicting COVID-19 Disease Course
Approach
Epigenetic immunophenotyping was performed on whole blood collected from unvaccinated patients who were hospitalized for COVID-19. Various immune cell populations (CD3, CD4, CD8 and regulatory T cells; natural killer (NK) cells; and naïve and memory B cells) were quantified. Measurement results were compared to healthy controls and analyzed for utility in predicting COVID-19 disease course. Disease stage was assigned according to Robert-Koch-Institute classification and disease course was characterized as:
- Poor prognosis – A change in disease stage from moderate to severe or critical or from severe to critical
- Good prognosis – A change in disease stage from severe or critical to moderate or from critical to severe or moderate or stably moderate
Epigenetic immunophenotyping was also performed on nasopharyngeal swab samples to evaluate whether these non-invasive sample types could be used for epigenetic immune monitoring.
Results
Epigenetic immunophenotyping of whole blood and nasopharyngeal swab samples showed significantly lower CD3 T cell counts and higher neutrophil counts in patients compared to controls. High CD3 T cell count and higher lymphocyte-to-neutrophil ratio (LNR) correlated positively with favorable clinical outcomes.
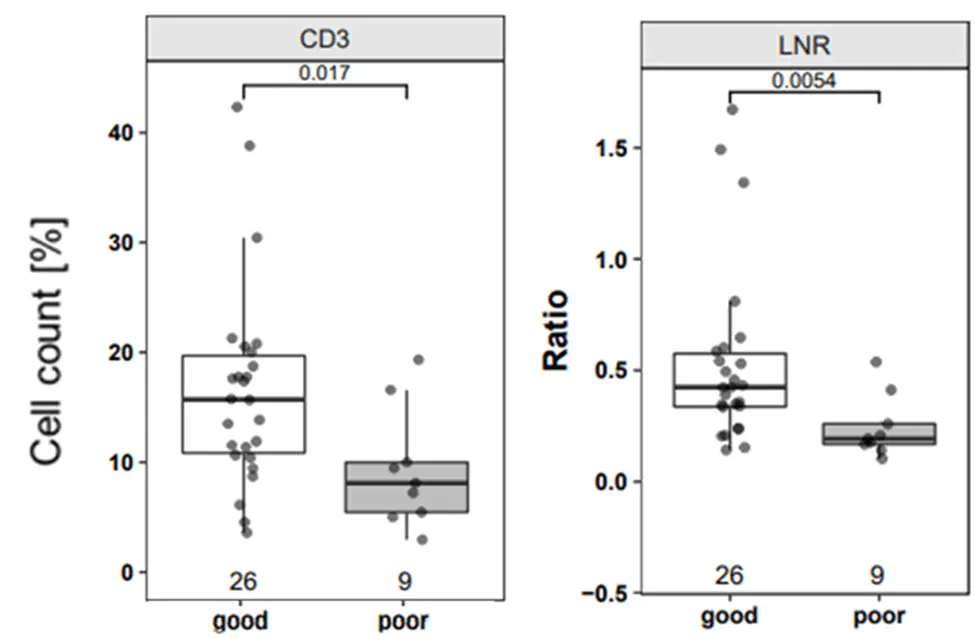 Figure 2. CD3 T cells and Lymphocyte-to-Neutrophil Ratio as Strong Prognostic Markers for Disease Course
Figure 2. CD3 T cells and Lymphocyte-to-Neutrophil Ratio as Strong Prognostic Markers for Disease CourseAssessing SARS-CoV-2 Vaccine Response
Approach
Dried blood spots were collected from healthy subjects before and after SARS-CoV-2 booster vaccination to assess immune cell population changes associated with vaccine response. Post-vaccination samples were taken at 6 hours, 18 hours, 24 hours, 48 hours and 6 days after vaccination.
Results
Epigenetic immune cell counting was performed for CD3 T cells, CCR7+, CTLA4+, TIGIT+, B cells, memory B cells, and IgM+ B cells and showed:
- T cell responses as early as 6 hours post vaccination
- Decreases in CD3 T cells, CCR7+ cells, and TIGIT+ cells after vaccination, with the most significant reduction in TIGIT+ cells
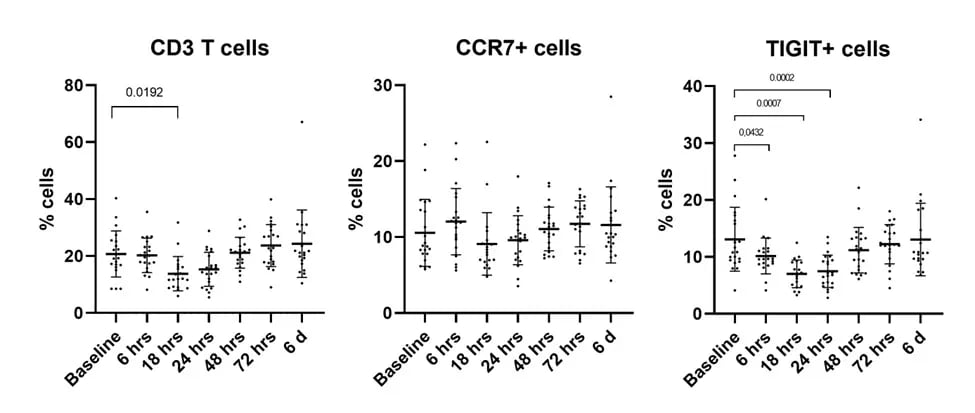 Figure 3. Decreases in Immune Cell Subsets After SARS-CoV-2 Vaccination
Figure 3. Decreases in Immune Cell Subsets After SARS-CoV-2 VaccinationKey Takeaways
Epigenetic immunophenotyping allows lymphocyte quantification from a range of sample matrices, including nasopharyngeal swabs and DBS, which can be performed by untrained individuals in a point-of-care or home setting.
The results demonstrated that epigenetic immunophenotyping has potential for predicting the disease course based on a patient’s level of CD3 T cells and neutrophils. Furthermore, epigenetic immunophenotyping allows for close monitoring of COVID-19 vaccination response with immune cell changes observable as early as 6 to 24 hours after vaccination.