The first observations of immunotherapy date back to 430 B.C. when Thucydides noticed that survivors of the plague outbreak developed resistance to the disease. Today, there are nearly 4000 immunotherapies in development, with over 1800 new agents added to the immuno-oncology (I-O) pipeline from 2017 to 2019.1 Despite the promise of immunotherapy, many patients become resistant or fail to respond. Consequently, clinical development is increasingly focused on combining novel I-O agents with other cancer treatments, including targeted drugs, chemotherapy, radiation, and other I-O therapies. It is for this and other reasons that clinical immune monitoring has become increasingly critical.
Importance of Immune Monitoring
A critical component of I-O studies, immune monitoring is essential for understanding, predicting, and monitoring the immune system response to therapeutic interventions. In clinical trials, immune monitoring approaches generally focus on 3 main areas2:
- The tumor and its microenvironment. Common parameters include presence and frequencies of immune cells such as CD8+ T cells or dendritic cells; expression levels of marker genes or proteins such as PD-L1, signaling changes; and genomic analyses such as T cell receptor (TCR) clonality, tumor mutational burden, microsatellite instability, or mismatch repair deficiency
- Peripheral blood. Analyses include lymphocyte counts, TCR clonality, serum PD-L1 levels, and circulating tumor DNA
- Clinical status. Clinical parameters that can be monitored include immune-related adverse events, microbiome composition, and prior therapies
Given the complexity of the immune response, no single approach to immune monitoring is sufficient for providing a comprehensive view of the immune system. Multiple methods will likely need to be used in concert to obtain a more complete picture.
An Epigenetic Approach to Immune Monitoring
Epiontis ID is a next generation method of immune monitoring based on measuring cell type-specific epigenetic markers that identify uniquely demethylated regions on genomic DNA, which is highly stable. Epigenetic modification of DNA by methylation is a mechanism of controlling gene expression. Epiontis ID exploits these (de)-methylation phenomena to quantitate immune cell populations using quantitative polymerase chain reaction-based assays that provide highly reproducible data (see Figure 1). This high reproducibility enables researchers to run measurements in flexible batches in parallel to their clinical studies, which can streamline logistics and reduce overall cost without sacrificing data quality.

A significant advantage of epigenetic immune cell quantification is that it can be applied to fresh, frozen, or paper-spotted dried blood and other bodily fluids or tissues, eliminating the need for special handling and making it possible to perform additional assays at a later date. Currently, Precision for Medicine offers over 30 pre-validated assays on the Epiontis ID platform, representing a mix of common surface markers such as CD4, CD8, and FoxP3, and lesser-known or newly discovered markers (see Figure 2). Any combination of these assays can be run on a single sample, without the need for any project-specific validation.
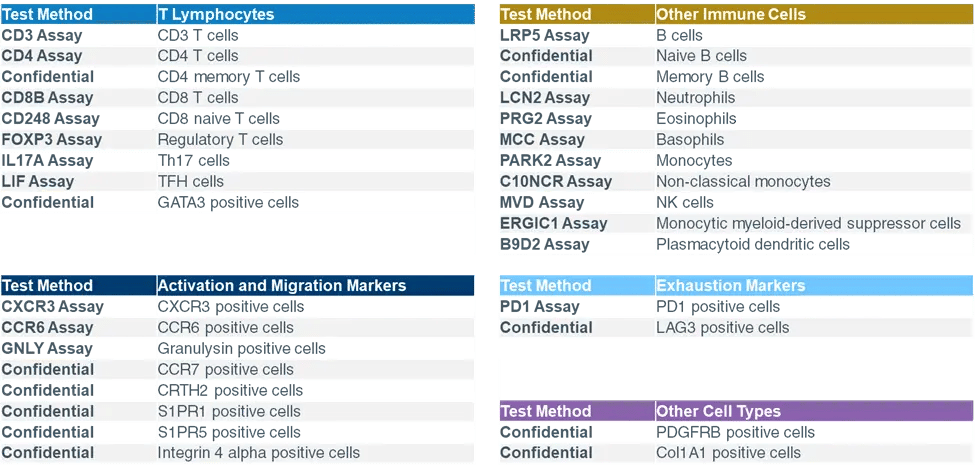 Figure 2. Fully customizable Epiontis ID panels, as of Q1 2021
Figure 2. Fully customizable Epiontis ID panels, as of Q1 2021Case Study: Epiontis ID
In a Phase 1a study of etigilimab, an anti-T cell immunoreceptor with Ig and ITIM domains (TIGIT) antibody, epigenetic immune monitoring was applied to regulatory T cells (Tregs) and CD8+ T cells. TIGIT is an immune checkpoint receptor that has been shown to inhibit T cell and natural killer (NK) cell activation and suppress the anti-cancer immune response. In addition to inhibiting TIBIT signaling, the anti-TIGIT antibody was hypothesized to deplete TIGIT-high Tregs via antibody-dependent cellular cytotoxicity.3
Peripheral immunophenotyping was performed using both flow cytometry and Epiontis ID, with a focus on the balance of Tregs and CD8+ T cells. Both methods demonstrated that treatment with the anti-TIGIT antibody reduced Treg frequency (see Figure 3) but did not significantly change CD8+ T cell frequency.3
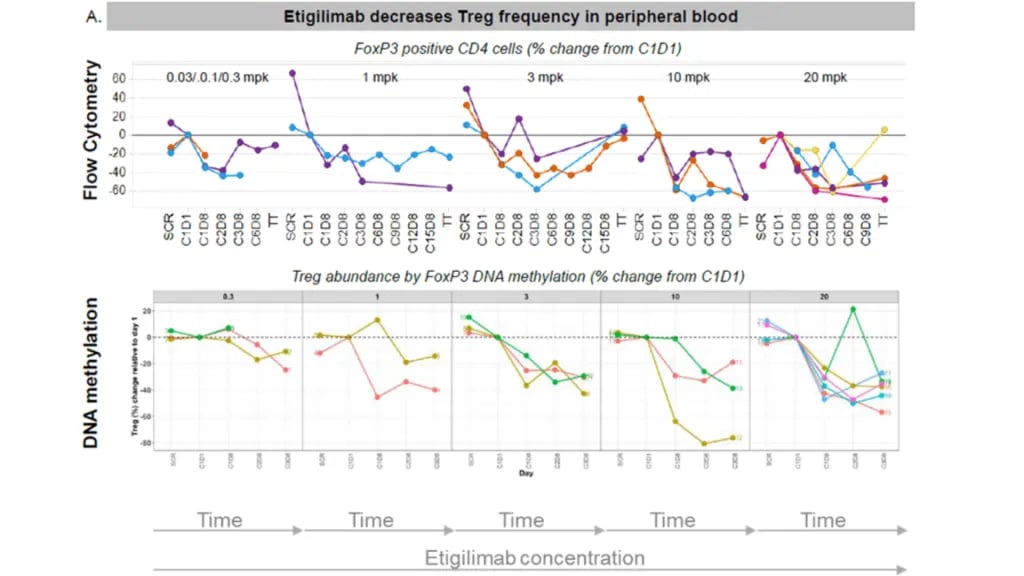 Figure 3. Etigilimab decreases Treg frequency in peripheral blood
Figure 3. Etigilimab decreases Treg frequency in peripheral bloodEpiontis ID and Flow Cytometry: Complementary Technologies
Epiontis ID and flow cytometry should be considered complementary, not competing technologies. Project type and study phase both factor into the determination of which technology is most appropriate for a particular clinical trial.
The strength of flow cytometry lies in the availability of more than 1000 labelled antibodies and the capability of multiplexed measurements, offering virtually unlimited combinations and potential definitions of gates and cell populations. Flow cytometry is well-suited for research, preclinical, and small early phase studies. It is also necessary for specialty applications such as receptor occupancy or protein phosphorylation.
Epiontis ID is distinguished by its very low sample requirement, logistical convenience, sample stability, standardized process, and off-the-shelf validated assays. It can be added for bridging in phase 1 and may be preferred in large scale, multi-center, late-stage trials due to its logistical convenience. Epiontis ID may also be more suitable for studies that require the measurement of blood and tissue in parallel.
Precision for Medicine offers multiple technology options for addressing any scientific question, thus allowing clinical trial requirements to inform selection of the optimal solution. Our goal is to maximize data generation with minimum sample requirements, easing the burden on both study participants and site staff without impacting data quality.
Webinar: Epiontis ID Immune Monitoring in Oncology Clinical Trials
For more detailed information on how Epiontis ID can be used in oncology clinical trials, watch the webinar:
Next Generation Immune Monitoring in Immuno-oncology Trials >
References
1. Yu JX, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18(12):899-900.
2. Axelrod M, Johnson DB, Balko JM. Emerging biomarkers for cancer immunotherapy in melanoma. Semin Cancer Biol. 2018;52(2):207-215.
3. Huang Y, Brunner A, Cai S, et al. Interim biomarker analysis of etigilimab (OMP-313M32), an anti-TIGIT antibody, in advanced solid tumors supports TIGIT-associated mechanisms of action. Society for Immunotherapy of Cancer, Washington, DC 2018 / Keystone 2019.