Protecting data integrity is a top priority
Studies may undergo transition because of acquisition at the Sponsor level, performance or capability issues, or study re-design. In such rescue scenarios, where study conduct is already underway, it is critical to recognize the unique challenges a study team may face. Seamless transition of administration, logistics, technology, and project and data management requires meticulous planning.
This planning must consider the needs of all stakeholders—the Sponsor, CRO, service providers, and sites. In this article, we discuss three key factors for effective, efficient transition of a study from a data management perspective.
I. Establish and maintain communication
Clear communication between the Sponsor and the Lead Data Manager of the incoming CRO is vital to the success of any study transition. Understanding preferences and setting expectations around the cadence, format, and content of communications at the outset builds trust, particularly if communication was a pressure point with the outgoing CRO.
-

Clinical Trials - Clinical Trial Strategy - Clinical Data Management - Clinical Biostatistics
Best Practices for Database Transfer in a Rescue Study
- |
II. Develop a transition plan and timeline
Thorough planning and preparation for data management activities are paramount to a successful transition. As soon as a scope of work and budget have been defined, the CRO should request key documents, including all versions of the protocol and copies of the data management plan, data review plan, case report forms (CRFs), annotated CRFs (aCRFs), configuration settings, edit check specifications, CRF completion guidelines, coding licensure information, local laboratory reference ranges, if applicable, and external vendor contact information. The CRO should then create a draft transition plan and timeline, which will help to identify areas for discussion during a formal kickoff meeting with the Sponsor.
During the kickoff meeting, the Sponsor may also find it useful to share why the study is being rescued and what concerns they had with their outgoing CRO. Another important topic for discussion is what to do with the existing database. Knowing the answers to these questions helps clarify what is expected of the data management function and provides the details necessary for finalizing the transition plan and timeline.
- What type of database is it?
- Will transferring the database URL be straightforward?
- Who has administrative responsibilities for the database?
- Does the Sponsor wish to transfer the data to an entirely new database?
- Are there randomization, drug dispensation, or other database modules that need to be transitioned?
- Does the Sponsor have a database transition date in mind?
- Is there an upcoming interim analysis or safety review meeting that requires a data cut?
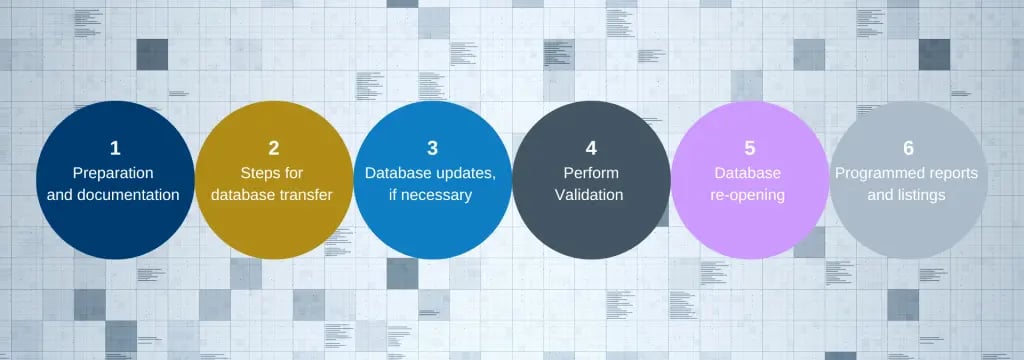 If new tasks are identified, the transition plan, timeline, and transition checklist should all be updated and distributed to the Data Management and Database Programming teams in a timely fashion.
If new tasks are identified, the transition plan, timeline, and transition checklist should all be updated and distributed to the Data Management and Database Programming teams in a timely fashion.
A transition checklist is a much-needed tool for guiding the Data Management and Database Programming teams through the steps required for the transition. This transition checklist should reflect tasks identified in the scope of work and transition plan, as well as responses to the questions asked during the kickoff meeting. If new tasks are identified, the transition plan, timeline, and transition checklist should all be updated and distributed to the Data Management and Database Programming teams in a timely fashion.
III. Complete the database transition and go live
Once the database transition is complete, the Lead Data Manager and Data Management and Database Programming teams move into the next phase of database maintenance and data cleaning and management. Shortly after the database goes live, programming of the listings, metrics, reconciliations and ad-hoc reports using data from the transitioned database begins.
Throughout the transition, new documents—including the transition plan, data management plan, CRFs, aCRFs, edit check specifications, and data transfer specifications—are created. Fully executed copies of these documents are filed in the project Trial Master File.
-

Clinical Trials - Data Intelligence - Clinical Trial Strategy - Clinical Biostatistics
Inside a CRO: The Critical Role of Clinical Data Managers in Clinical Research
- |
Key Takeaway
A study transition built upon a foundation of solid communication, detailed planning, and rigorous documentation sets the stage for a successful rescue. At Precision for Medicine, we have extensive experience rescuing and transitioning data management activities and databases.




