CASE STUDY
Characterization of Specific EGFR Mutations in CTCs Isolated by ApoStream® in NSCLC Patients
Clinical Application: Exploring CTCs as an alternative to tissue biopsy specimens to monitor mutations for optimization of targeted therapy
Key Words: CTCs, ApoStream®, LSC, mutational analysis, correlative study, NSCLC
Background: Circulating tumor cells are often touted as a potential surrogate for tissue biopsies when studying the biology of an individual patient’s cancer. Obtaining biopsy samples is an invasive process, and not always possible depending on the state of the patient’s cancer. Access to a blood-based mechanism of understanding tumor biology is one that appeals to many clinicians; however, much research still needs to be conducted to establish the clinical relevance of CTCs as a true alternative to biopsy. This study was structured as a means of examining that claim. CTCs were isolated from the blood of non-small cell lung cancer patients using ApoStream®, and the status of exons 19 and 21 on the EGFR gene were examined by means of ICE-COLD PCR. Results from this mutational analysis were then compared to mutational analysis done on biopsy specimens from the same patient.
Methods: Two tubes of blood were collected from 36 NSCLC patients and CTCs enriched from each using ApoStream®. Following capture, CTCs from one tube were placed on slides and stained for cytokeratin (CK), CD45, and DAPI. Stained cells were analyzed using Laser Scanning Cytometry (LSC) and cells exhibiting a CK+/CD45-/DAPI+ phenotype enumerated as CTCs; additional phenotypes examined included CK-/CD45-/DAPI+ and CK+/CD45+/DAPI+ cells. DNA was extracted from the cells from the second tube, then undergo ICE-COLD PCR to analyze the mutational status of the EGFR gene, and the results were compared to mutational analysis conducted on archived biopsy specimens.
Results:

Figure 1. ApoStream® Results. Median counts of both CTCs and putative CTCs (CK- /CD45-, CK+/CD45+) are reported here. Healthy donor samples (n=3) were also analyzed; CTCs were not detected in any samples. Comparatively low counts were obtained for both CK-/CD45- (median: 107, range: 28 – 457) and CK+/CD45+ (median: 15, range: 7 – 26) cells.
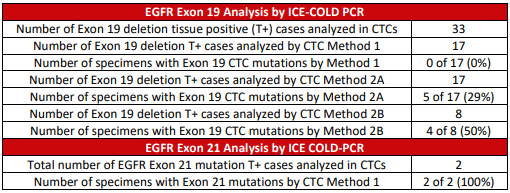
CTCs from tissue-positive patients from 0% with standard ICE COLD-PCR to 27% and 50% with methods 2A and 2B. Only a portion of the extracted template was tested per PCR, which might
have led to discrepant results.
Impact:
ApoCell has demonstrated that ApoStream® is capable of successfully isolating CTCs and putative CTCs from the blood of NSCLC patients. Analysis of EGFR mutations and comparison with mutation data from tissue biopsies enables exploration of the clinical utility of CTCs as an alternative to tissue biopsy.
Reference: Roarty EB, et al. “Characterization and Identification of Specific EGFR Mutations in Circulating Tumor Cells (CTCs) Isolated from NSCLC Patients Using an Antibody-Independent Method, ApoStream®: An Update Report.” NCI-AACR-EORTC 2013.